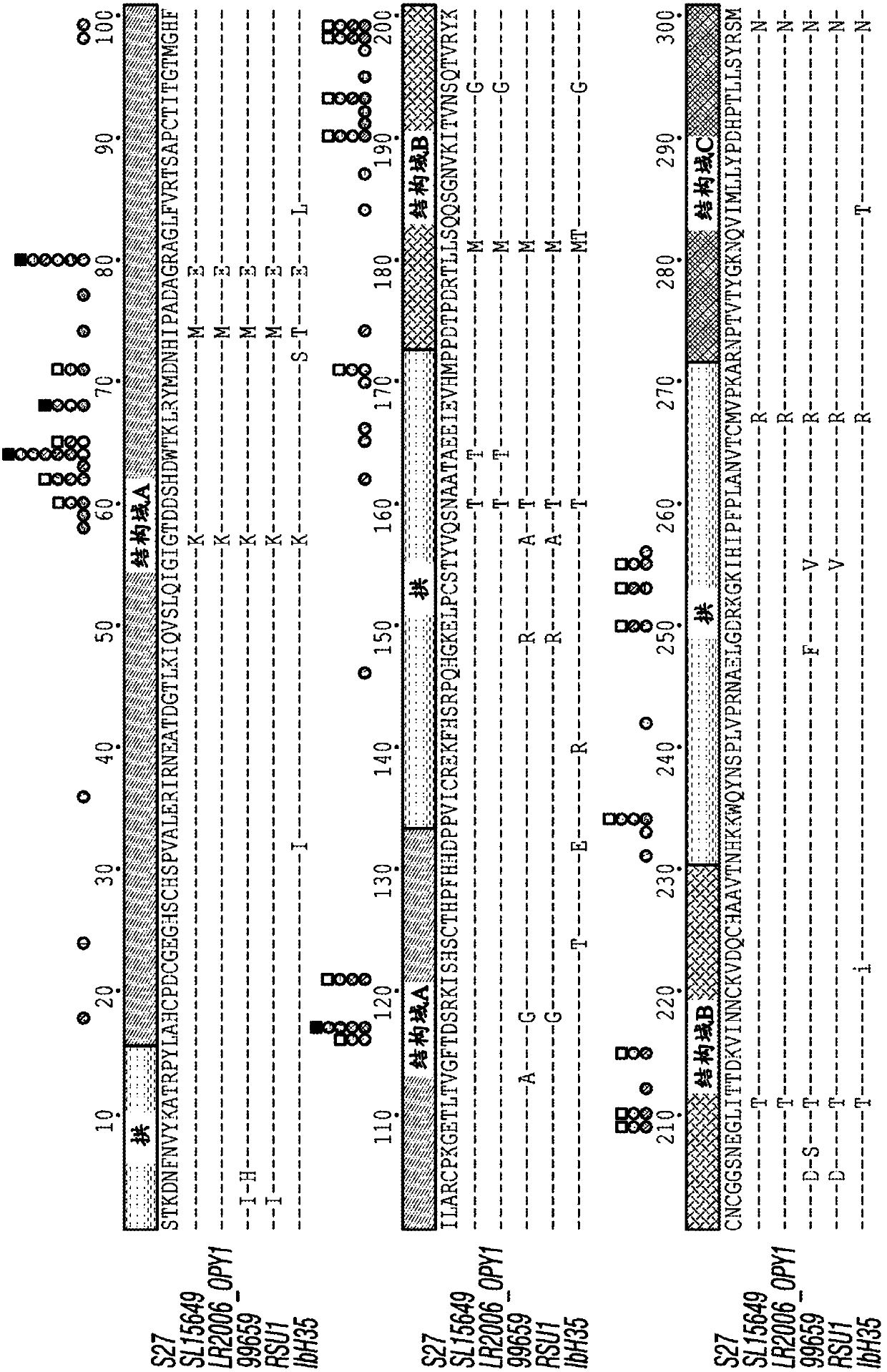
Antibody-mediated neutralization of chikungunya virus
A Kungunya fever, antibody technology, applied in the fields of medicine, infectious diseases and immunology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0191] Example 1: Materials and methods
[0192] Isolation of human mAbs. PBMCs were obtained from persons ~5 years after documented symptomatic CHKV infection in Sri Lanka. B cells were transformed in 384-well plates with EBV in the presence of CpG. Supernatants from the resulting B-cell lymphoblastoid cell lines were screened for the presence of human CHKV-specific binding antibodies by ELISA using live CHIKV vaccine strain 181 / 25 virus as antigen. Transformed B cells are harvested and fused into myeloma cell lines, distributed into culture plates and expanded, and selected by growth in hypoxanthine-aminopterin-thymidine medium containing ouabain. Cloning of hybridomas by single cell sorting. Supernatants from clonal hybridomas grown in serum-free medium were collected, purified from clarified medium by protein G chromatography and concentrated.
[0193] Neutralization assay. Purified IgG mAb proteins were tested for neutralizing activity using CHKV viral replicon parti...
Embodiment 2
[0224] Example 2 - Results
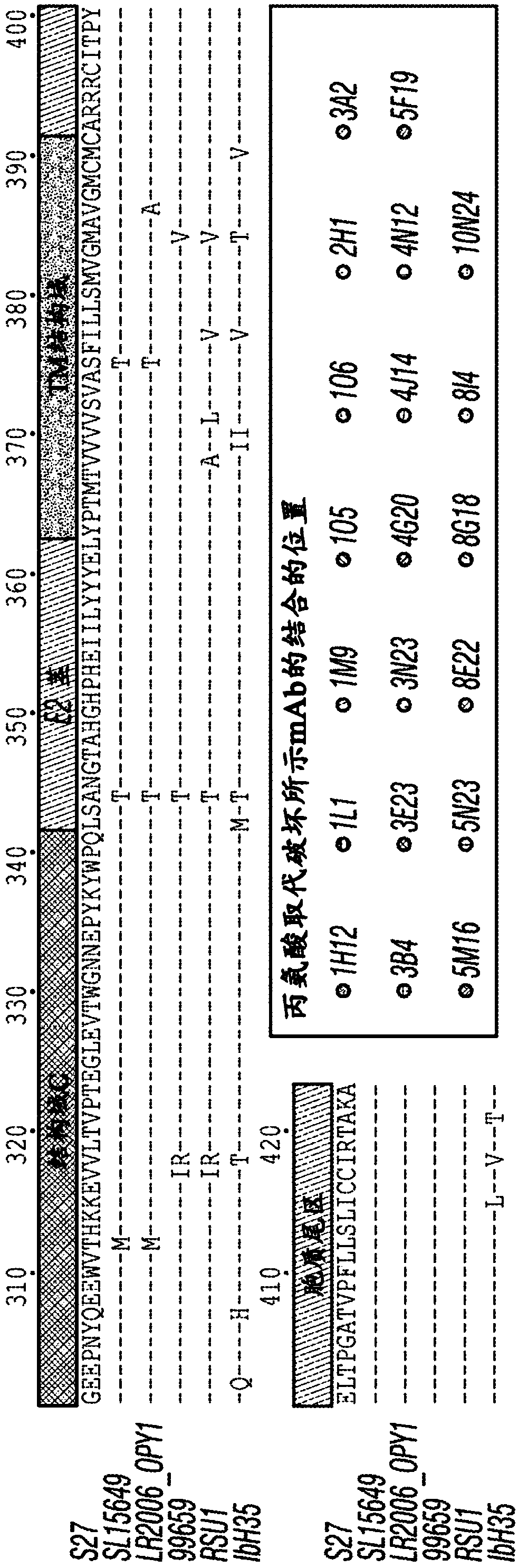
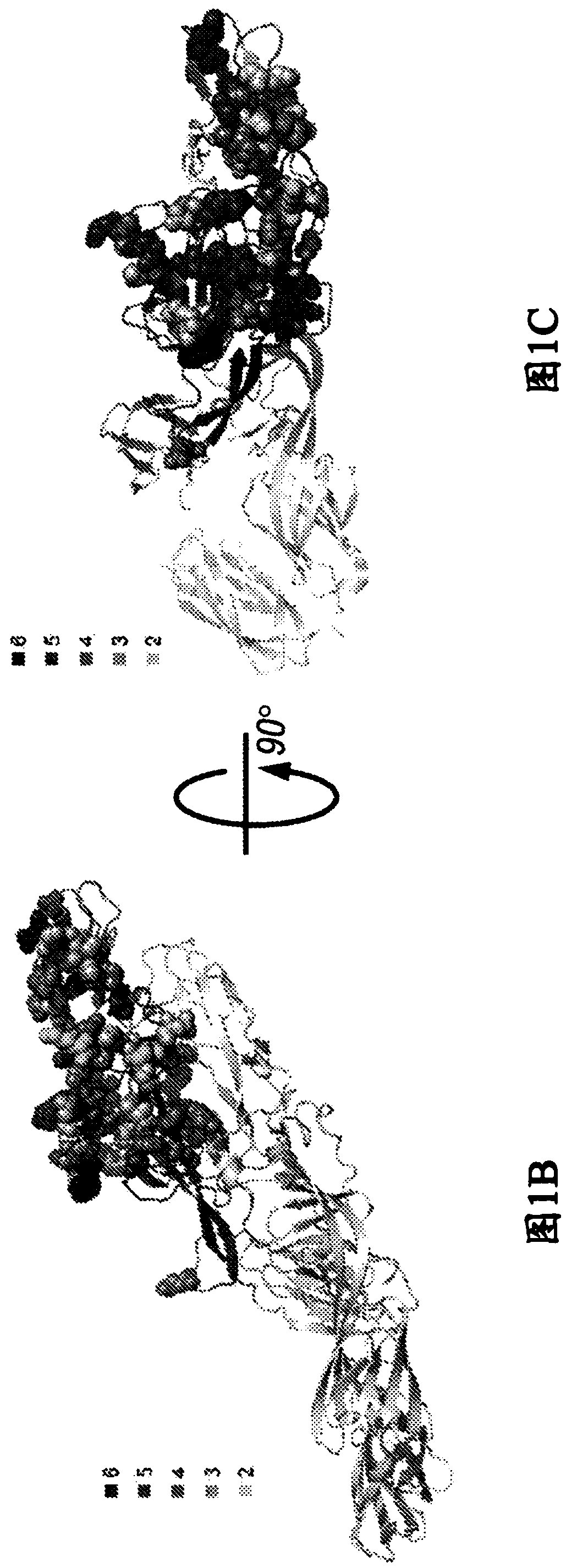
[0225] Isolation of CHIKV-specific human mAbs. The inventor of the present invention acquired CHIKV infection in Sri Lanka from 2006 and developed fever, arthralgia and rash ( Figure 4 ) isolated a panel of mAbs from a single individual. The online approach provides the clinical course and B cell transformation and screening program. They transformed B cells in a single blood sample collected from a donor five and a half years after natural infection in two separate experiments. They observed a virus-specific B cell frequency of approximately 1 in 1,000 total B cells and established 30 stable hybridomas from B cell lines secreting antibodies that bind to the virus. The mAb panel contained multiple subclasses of IgG with 24 IgGl, 3 IgG2 and 2 IgG3; one was not identified due to poor hybridoma growth (Table 5).
[0226] Evaluation of mAb neutralization. Eighteen mAbs exhibited neutralizing activity against the Asian CHIKV strain SL15649-GFP vir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com