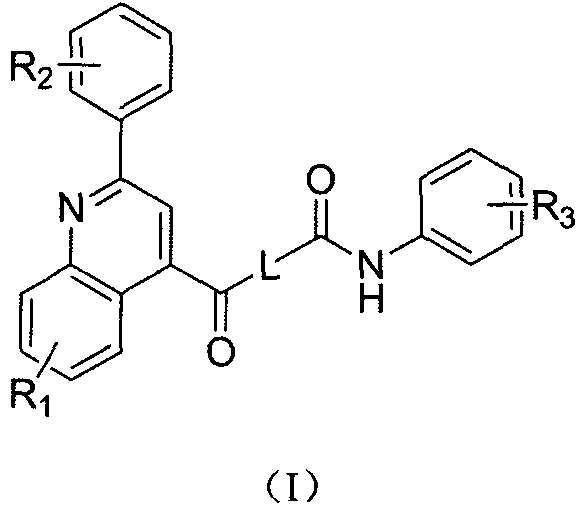
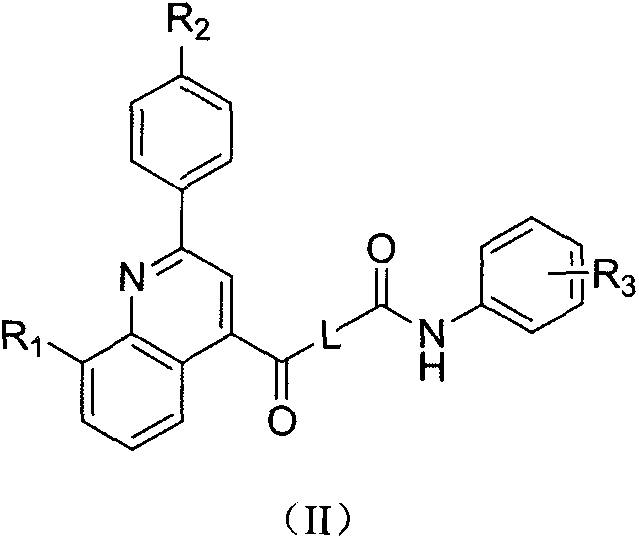
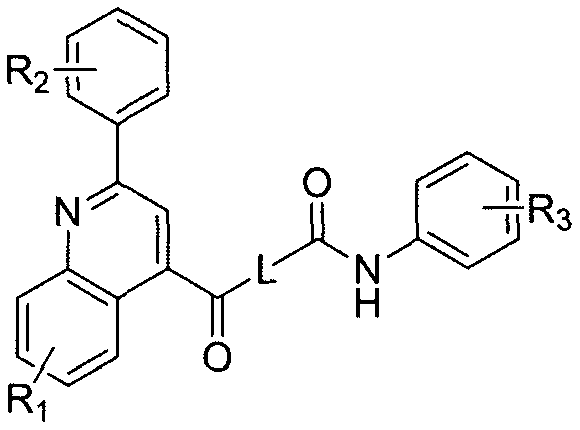
Phenylquinoline TRPV1 antagonist and its preparation method and application
A phenylquinoline and phenyl technology, applied in the field of medicinal chemistry, can solve the problems of loss of response to harmful stimuli, loss of pain, and burning sensation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Preparation of 2-phenylquinoline-3-carboxylic acid (iii)
[0104] Add KOH (0.56g, 10.02mmol) and absolute ethanol (10ml) into a 50ml single-necked bottle. After the KOH is completely dissolved, add isatin (i) (0.5g, 3.34mmol), stir at reflux at 80°C for 15min, and then add Acetophenone (ii) (0.82g, 6.8mmol), heat to reflux for 12h, remove the solvent under reduced pressure, add water (20ml) to dissolve, wash with saturated brine (20ml×3) take the water layer, adjust pH=2 with concentrated hydrochloric acid , and filtered to obtain 0.82 g of a light yellow solid, with a yield of 96.87%.
Embodiment 2
[0106]Preparation of N-phenyl-4-(2-phenylquinoline-4-carbonyl)piperazine-1-carboxamide (1)
[0107] (a) Preparation of isocyanatobenzene (v)
[0108] Dissolve aniline (1g, 10mmol) in DMSO (10ml), add N,N'-carbonyldiimidazole (2g, 12mmol) to the solution, stir at room temperature for 2h, add water (30ml) and ethyl acetate (30ml) , extraction and separation, the aqueous layer was washed with ethyl acetate (20ml×3), and after combining the organic layers for 24h, the solvent was evaporated under reduced pressure, and the solvent was evaporated under reduced pressure to obtain an oily substance;
[0109] (b) Preparation of N-phenylpiperazine-1-carboxamide (vii)
[0110] The product obtained in (a) was dissolved in dichloromethane (20ml), 1-Boc-piperazine (1.67g, 9mmol) was added, stirred at room temperature for 2h, and the solvent was evaporated under reduced pressure to obtain an oily substance. The matching ratio is petroleum ether: ethyl acetate = 4:1. The obtained product w...
Embodiment 3
[0115] Preparation of 4-(2-phenylquinoline-4-carbonyl)-N-(4-methylphenyl)piperazine-1-amide (2)
[0116] Referring to the preparation method of 1 in Example 2, compound 2 was obtained as a light yellow solid with a yield of 55.2%, mp: 105-109°C;
[0117] 1 H NMR (300MHz, DMSO-d 6 )δppm: 8.53(s, 1H, NH), 8.34(d, J=6.5Hz, 2H, Ar-H), 8.27-8.09(m, 2H, Ar-H), 7.86(t, J=7.3Hz, 2H, Ar-H), 7.76-7.46(m, 4H, Ar-H), 7.31(d, J=8.4Hz, 2H, Ar-H), 7.04(d, J=8.3Hz, 2H, Ar-H ), 3.97-3.60 (m, 4H, piperazine), 3.53-3.12 (m, 4H, piperazine), 2.22 (s, 3H, CH 3 ); 13 C NMR (75MHz, DMSO-d 6 )δppm:166.45,156.32,155.56,148.12,143.76,138.52,138.14,131.17,131.01,130.43,130.17,129.36,129.21,127.96,127.78,125.24,123.35,120.29,119.24,116.05,46.95,44.43,44.03,41.73 , 20.80; ESI-MS m / z: 451.5 ([M+H] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com