A kind of pharmaceutical composition for treating Parkinson's syndrome and its preparation method and application
A technology of composition and syndrome, which is applied in the field of pharmaceutical composition for the treatment of Parkinson's syndrome, can solve the problems of frozen stiffness attack or mental symptoms, deterioration of the end of the dose, adverse reactions, etc., and is suitable for large-scale production, improving symptoms, Excellent curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
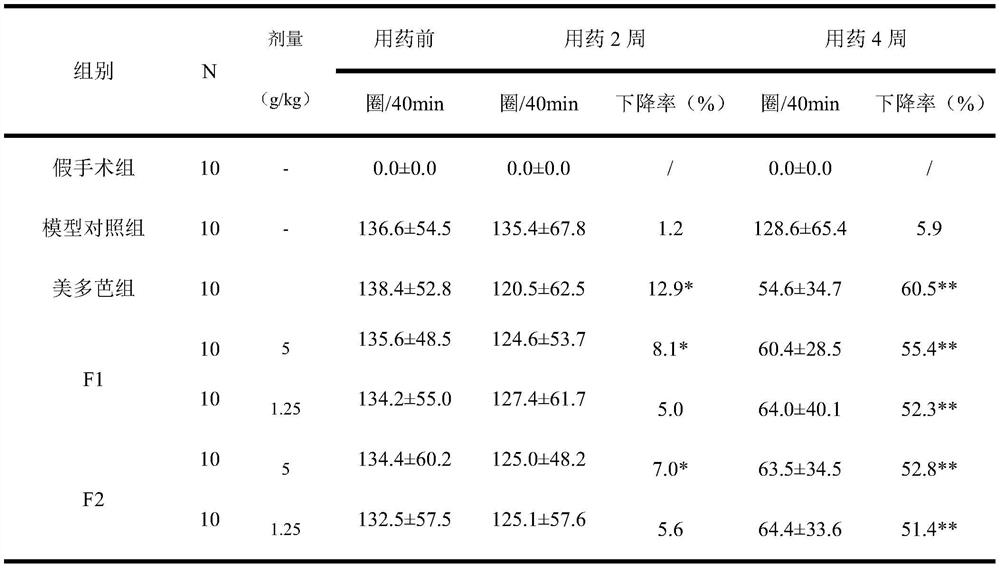
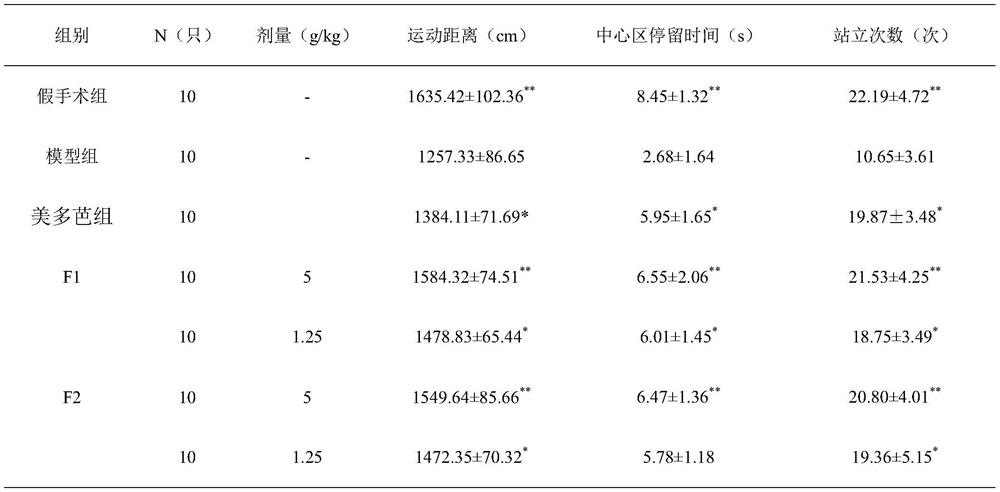
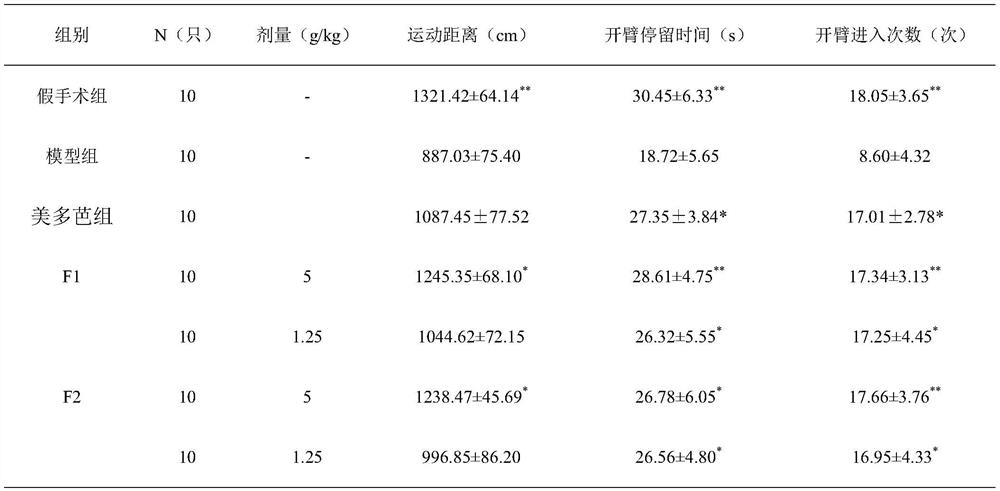
[0027] The preparation of embodiment 1 pharmaceutical tablet of the present invention
[0028] Weigh 10g of rehmannia glutinosa, 10g of Gastrodia elata, and 3g of scorpion, add 100g of auxiliary materials starch to granulate, 5g of magnesium stearate, 100g of dextrin, and 100g of microcrystalline cellulose, uniformly prepare granules, and press into tablets to obtain tablets.
Embodiment 2
[0029] The preparation of embodiment 2 pharmaceutical tablet of the present invention
[0030] Weigh 20g of rehmannia glutinosa, 20g of Gastrodia elata, and 5g of scorpion, add 120g of auxiliary materials starch to granulate, 5g of magnesium stearate, 110g of dextrin, and 110g of microcrystalline cellulose, uniformly prepare granules, and press into tablets to obtain tablets.
Embodiment 3
[0031] The preparation of embodiment 3 pharmaceutical tablet of the present invention
[0032] Weigh 30g of rehmannia glutinosa, 30g of Gastrodia elata, and 6g of scorpion, add 150g of auxiliary materials starch to granulate, 5g of magnesium stearate, 150g of dextrin, and 150g of microcrystalline cellulose, uniformly prepare granules, and press into tablets to obtain tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com