Treatment of beta-thalassemia using actrii ligand traps
A technology for thalassemia and activin receptors, which can be used in medical preparations containing active ingredients, blood diseases, pharmaceutical formulations, etc., and can solve problems such as unmet medical needs and untreated β-thalassemia.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0394] 8.2 Example 2: A phase 3 double-blind randomized placebo-controlled multicenter study to determine the efficacy and safety of ACTRIIB-HFC (SEQ ID NO: 25) in adults with transfusion-independent beta thalassemia
[0395] This example provides an overview of a phase 3 double-blind randomized placebo-controlled multicenter study to determine the efficacy and safety of ACTRIIB-HFC (SEQ ID NO: 25) in adults with transfusion-independent beta thalassemia. The indications for the phase 3 study are adults with transfusion-independent beta thalassemia, who are diagnosed with β-thalassemia or hemoglobin E / β-thalassemia.
[0396] 8.2.1 Goal
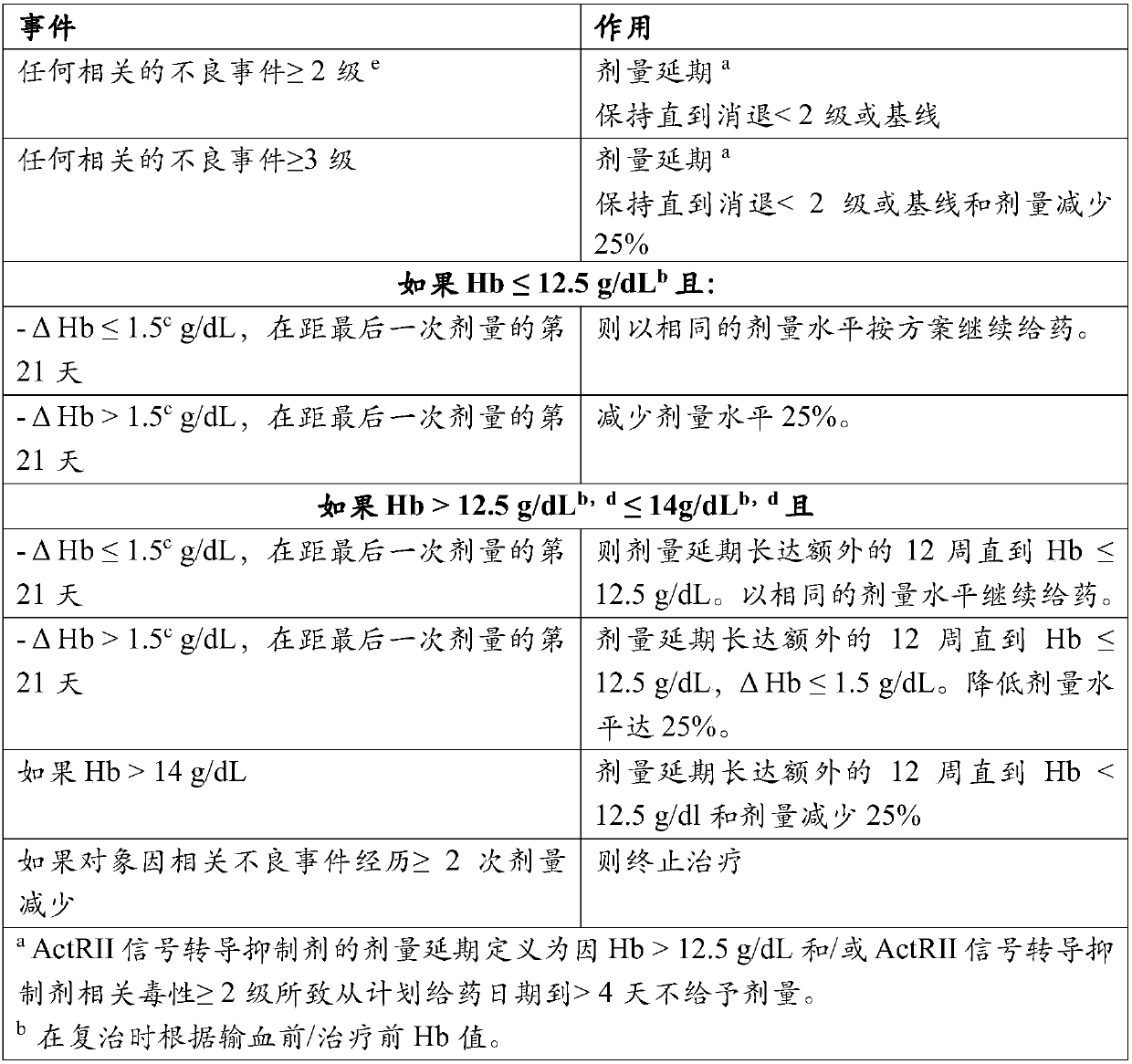
[0397] The main goal of the Phase 3 study is to determine if ActRIIB-hFc (SEQ ID NO: 25) is diagnosed as transfusion-independent β-thalassemia, documented β-thalassemia or hemoglobin E / β-thalassemia diagnosis, age ≥18 Years old and have received 0-6 red blood cell units and average baseline hemoglobin level within 24 weeks prior to random assignment ...
Embodiment 3
[0423] 8.3 Example 3: ACTRIIB-HFC (SEQ ID NO: 25) signal transduction inhibitor increases hemoglobin and reduces the burden of blood transfusion and reduces liver iron concentration in adults with β thalassemia
[0424] 8.3.1 Introduction
[0425] ActRIIB-hFc (SEQ ID NO: 25), a fusion protein containing a modified activin receptor, is being developed for the treatment of β-thalassemia. In β-thalassemia, anemia and complications are caused by ineffective erythropoiesis driven by excess α-globin. ActRIIB-hFc (SEQ ID NO: 25) binds to other ligands in the GDF11 and TGF-β superfamily to promote late red blood cell differentiation. Non-clinical and clinical studies have shown that ActRIIB-hFc (SEQ ID NO: 25) has a well-tolerated and corrective effect on ineffective erythropoiesis (Suragani R, Blood 2014, Attie K, Am JHematol 2014).
[0426] This example provides data from an ongoing Phase 2 multicenter open-label dose exploration study to evaluate ActRIIB-hFc (SEQ ID NO: 25) in adults wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com