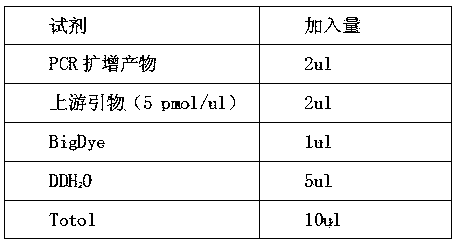
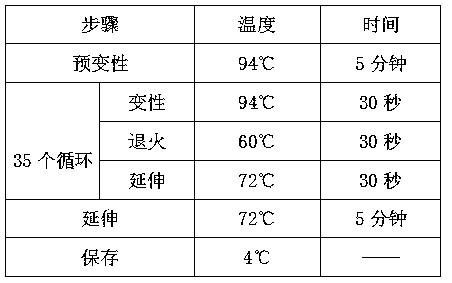
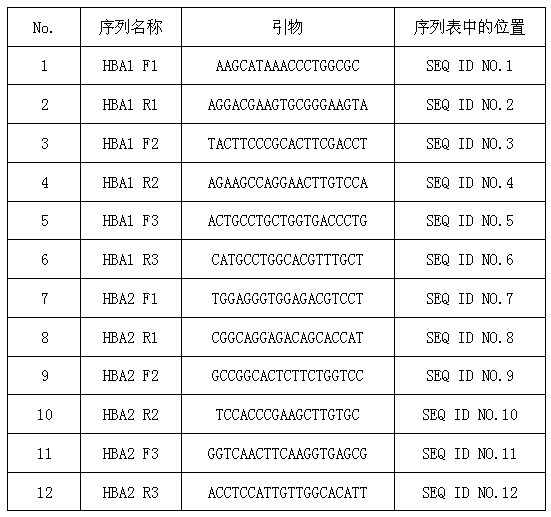
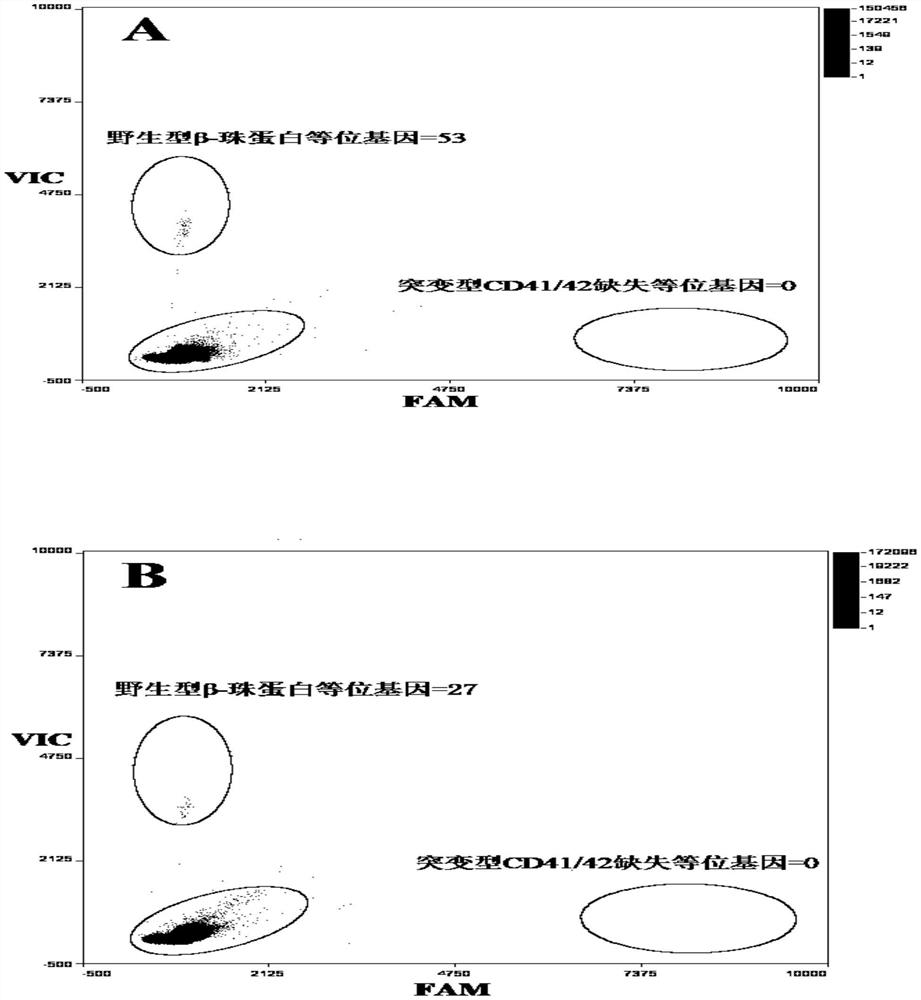
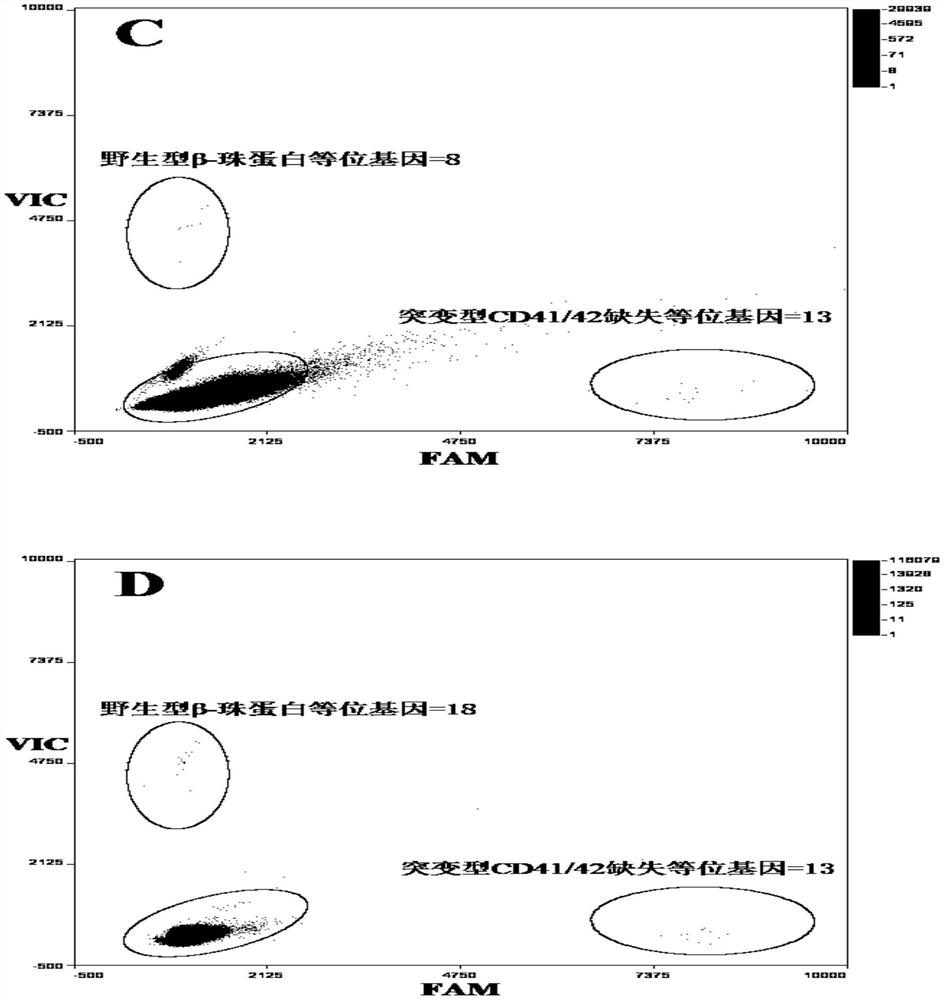
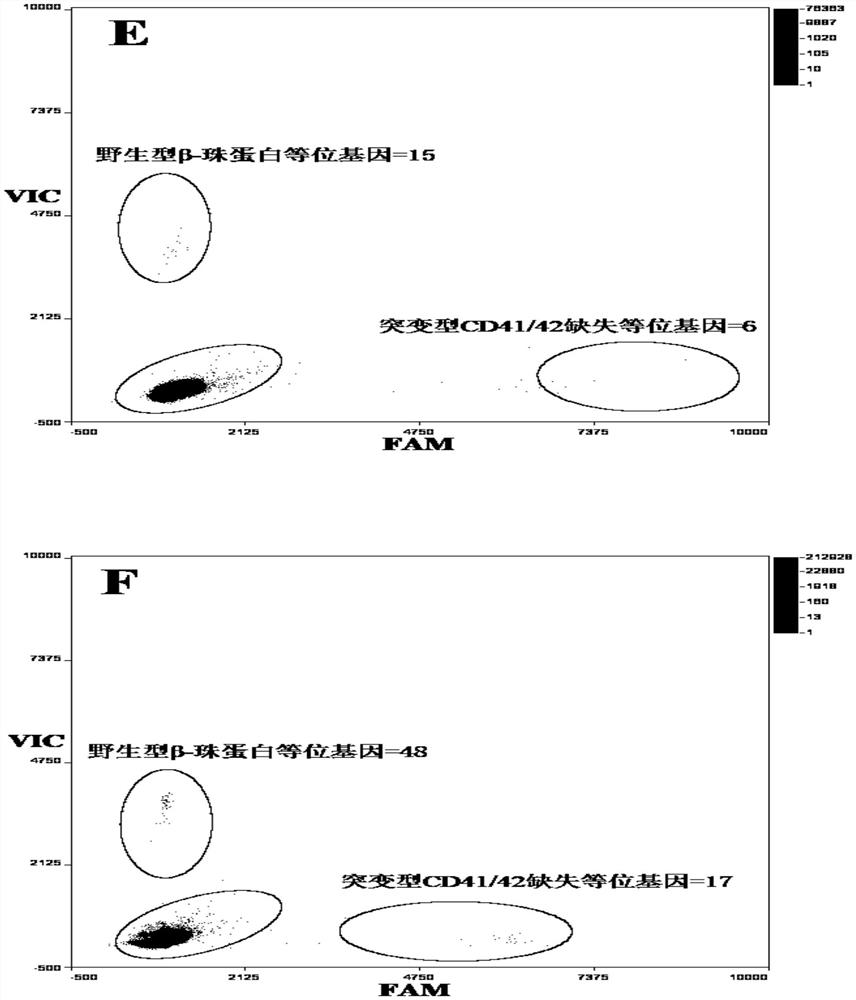
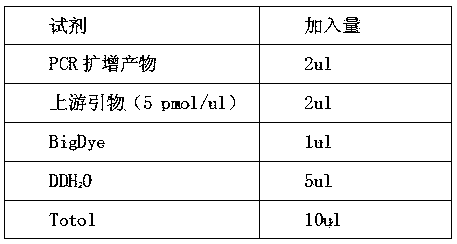
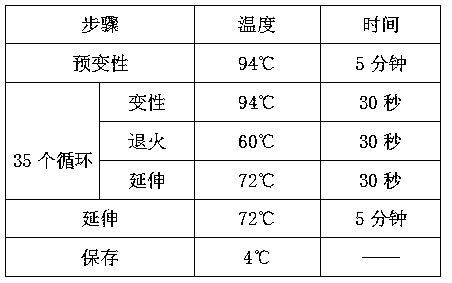
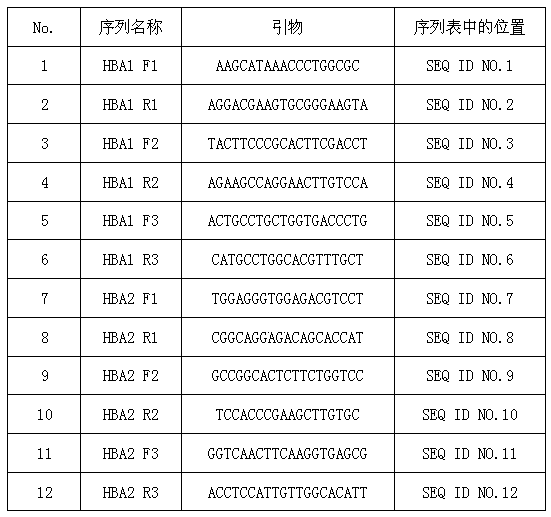
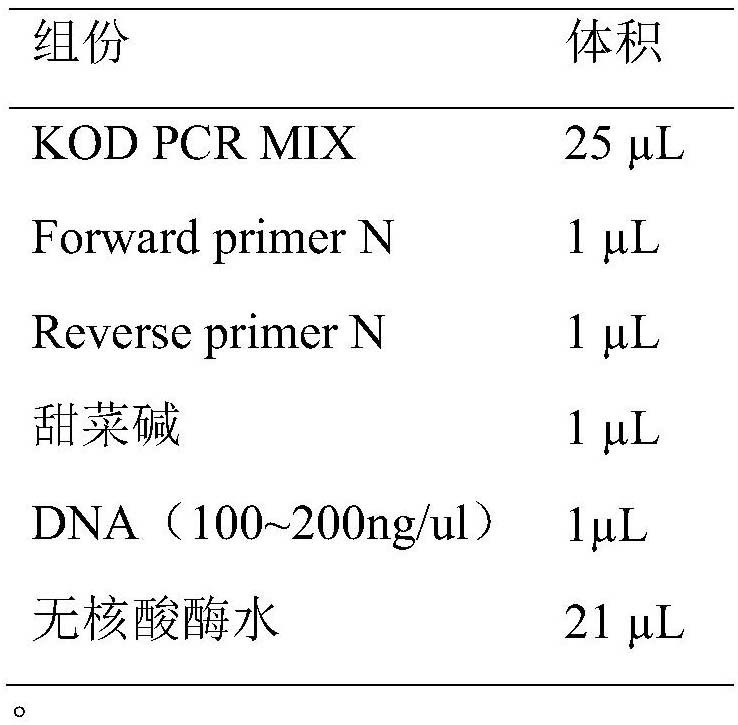
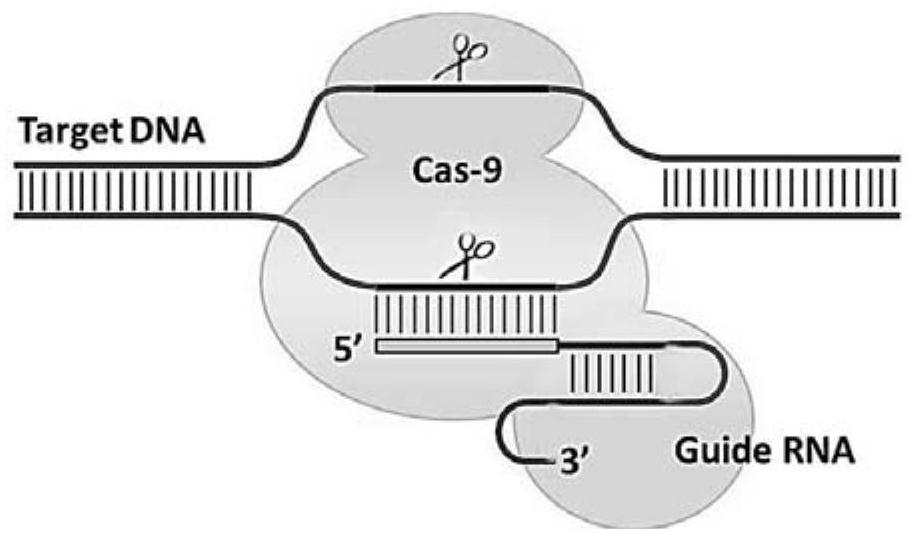
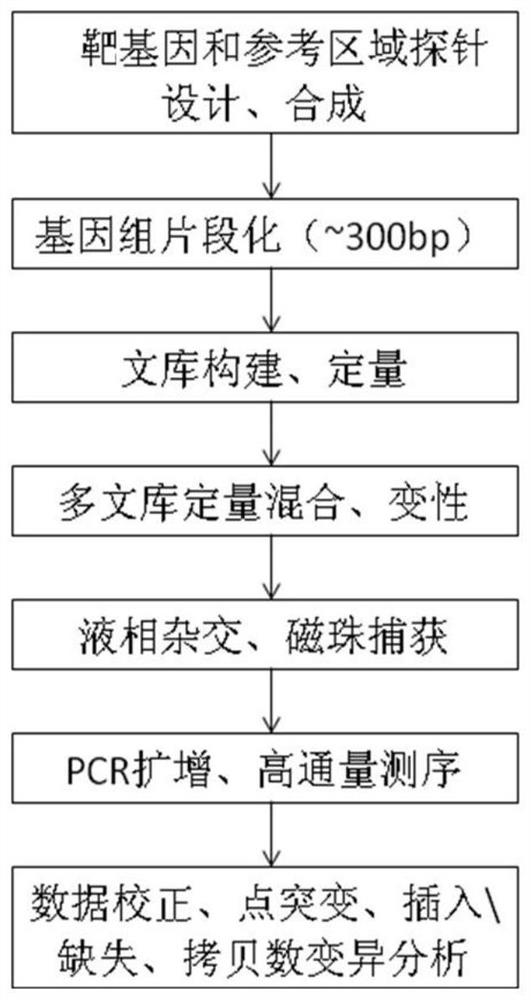
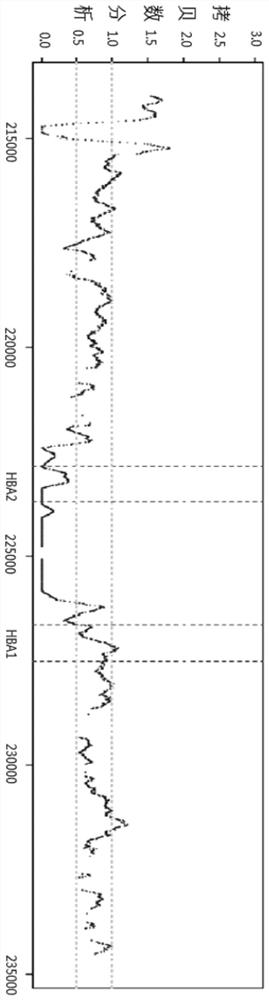
Patents
Literature
33 results about "Beta-thalassaemia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Alpha-thalassemia screening kit and application thereof in prenatal screening
ActiveCN103421903AReasonable primer designAchieving Prenatal ScreeningMicrobiological testing/measurementDiseaseObstetrics
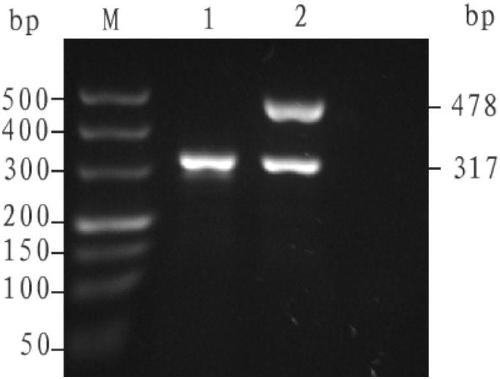
The invention discloses an alpha-thalassemia screening kit and application thereof in prenatal screening and belongs to the technical field of prenatal screening of alpha-thalassemia. The alpha-thalassemia screening kit comprises a negative control sample, a positive control sample, PCRmasterMIX, and a PCR primer. Application of the alpha-thalassemia screening kit includes: amplifying fetal DNA in maternal peripheral blood by the designed primer so as to perform prenatal screening on alpha-thalassemia. The maternal peripheral blood is collected for prenatal screening of alpha-thalassemia, with no need for puncturing the amnion cavity and inserting pile tissue and with no injury to fetus, and the alpha-thalassemia screening kit is safe and reliable; accuracy is up to 99.99%; the technical blank of noninvasive prenatal screening of alpha-thalassemia is filled, and fewer children with diseases are born.
Owner:邯郸市康业生物科技有限公司
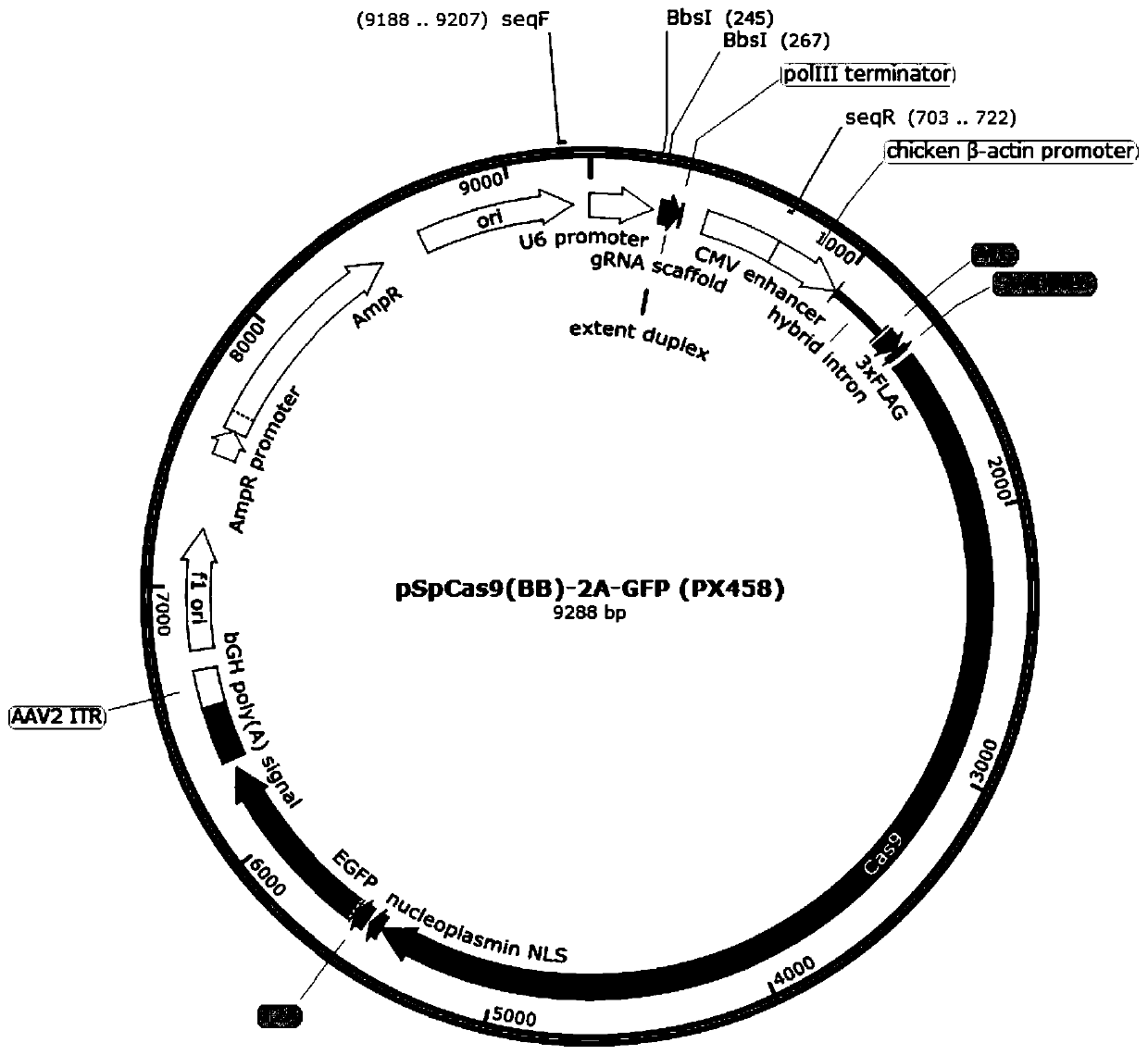
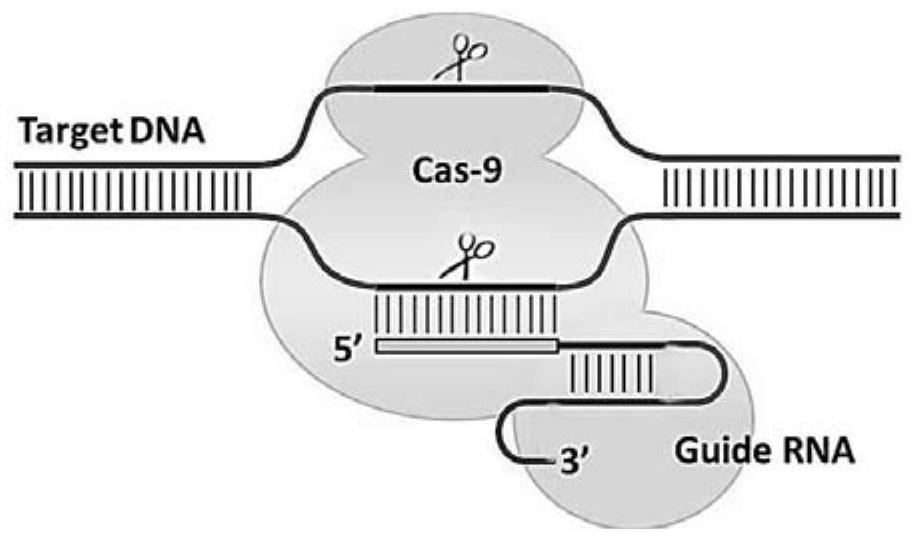
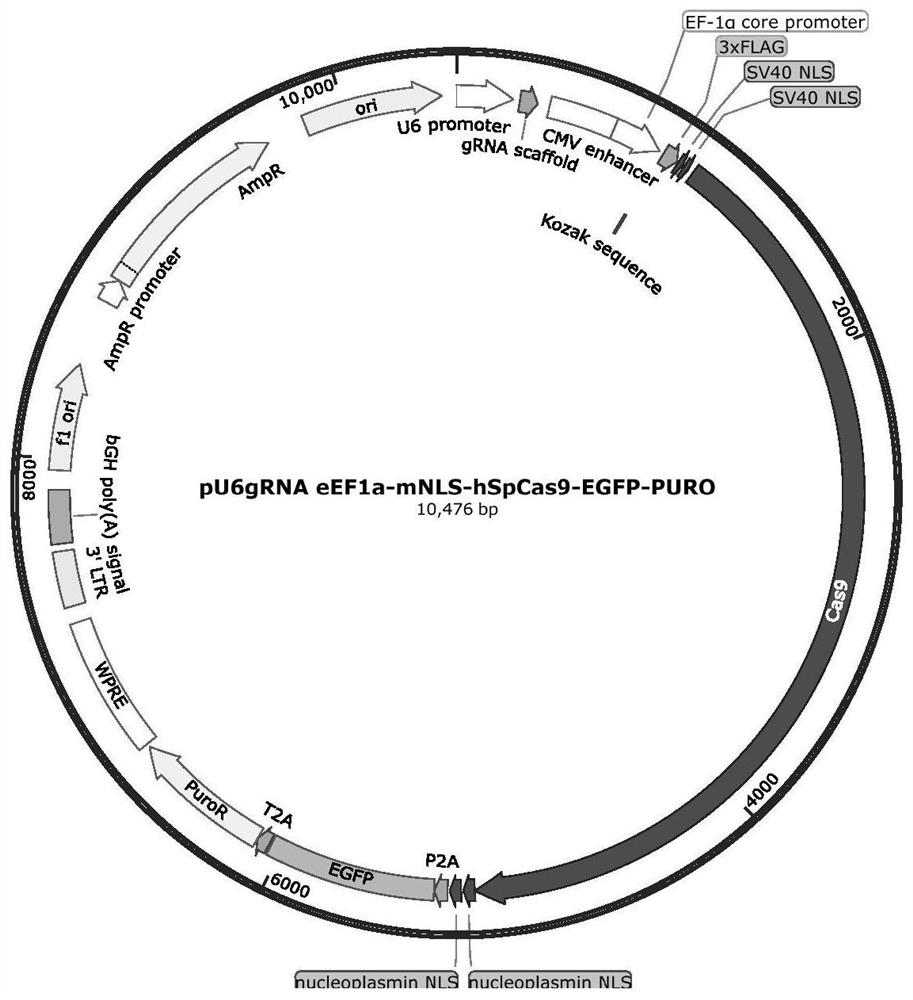
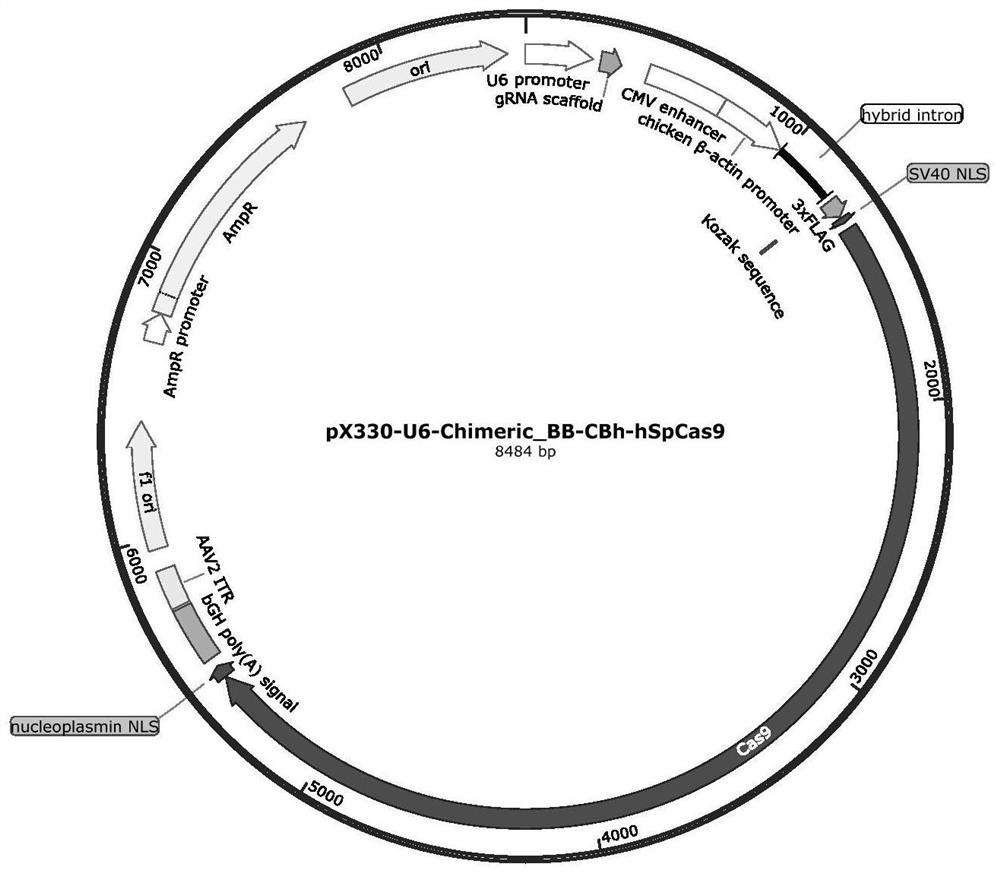
gRNA for knocking out BCL11A genes or BCL11A gene enhancers, gRNA composition and electrorotation method
PendingCN109706148AImprove cutting efficiencyImprove efficiencyGenetic material ingredientsStable introduction of DNAGenetic enhancementSickle cell anemia
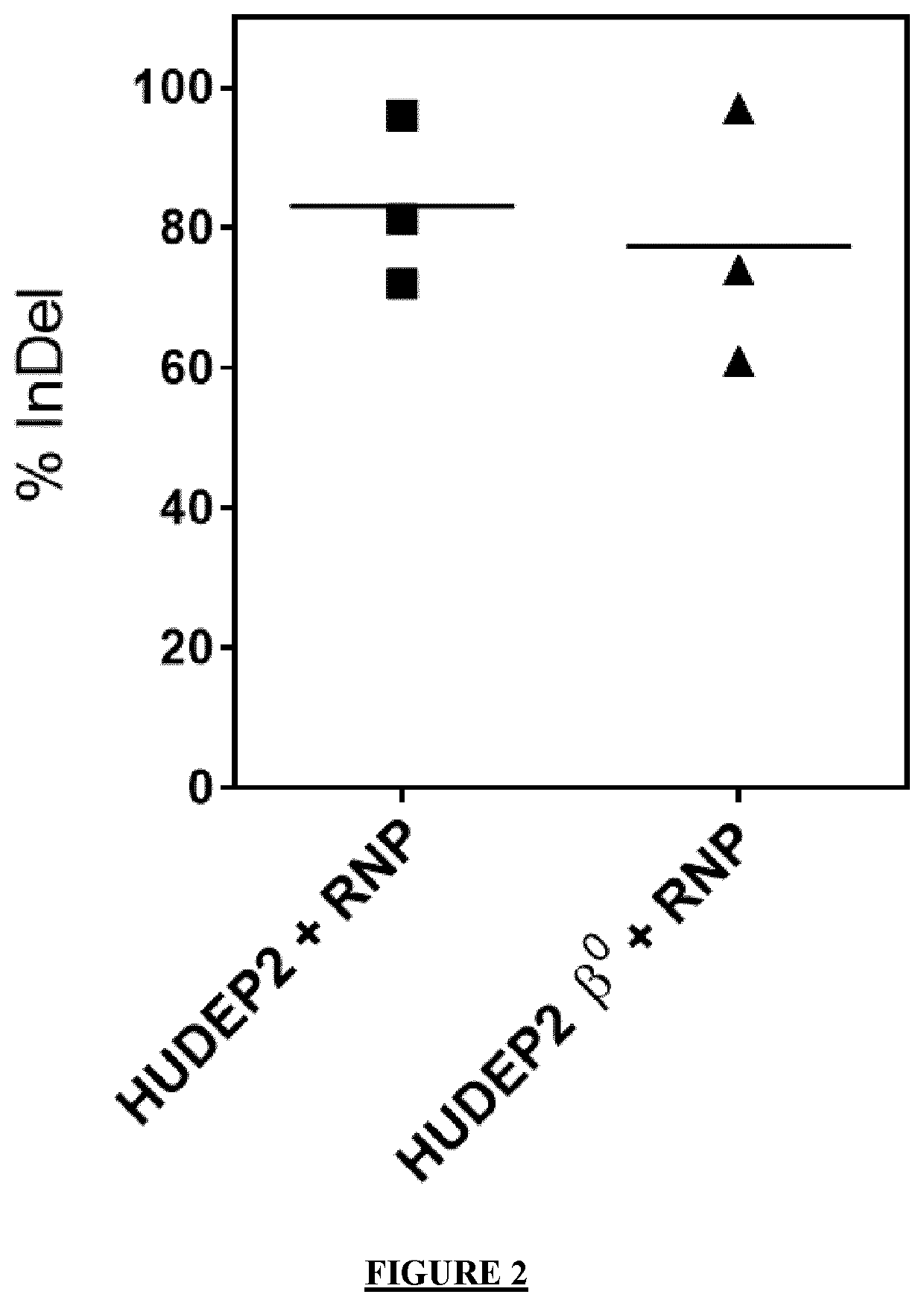
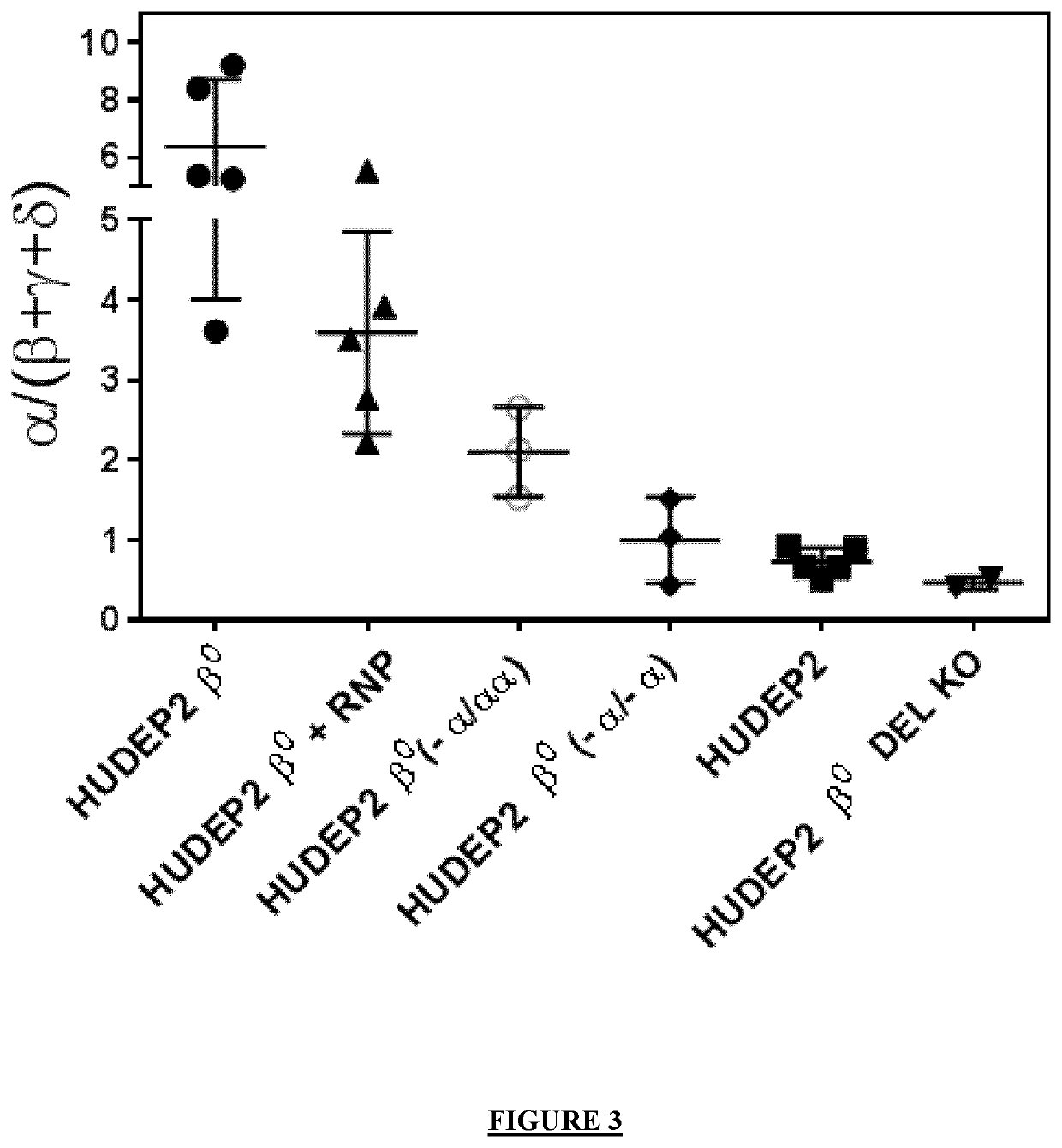
The invention provides gRNA for knocking out BCL11A genes or BCL11A gene enhancers, a gRNA composition, an expression vector, CRISPR-Cas9 RNP, a CRISPR-Cas9 RNP composition, a CRISPR-Cas9 system, an electrorotation method, a reagent kit and applications thereof. A CRISPR-Cas9 technique is adopted to perform targeted cutting on hemopoietic stem cells, so that the BCL11A gene expression amount is reduced, and the hemochrome expression quantity of fetuses is high. The technique is hopeful to become a new means for treating thalassaemia and sickle cell anemia. The CRISPR-Cas9 technique is adoptedto realize mutation of BCL11A genes. The design is simple, the use is convenient, the cost is low, and the efficiency is high.
Owner:广东赤萌医疗科技有限公司
Thalassemia-induced multipotent stem cell as well as preparation method and application thereof
ActiveCN102628029AEfficient manufacturingMicrobiological testing/measurementMammal material medical ingredientsInduced pluripotent stem cellGenome
The invention relates to a thalassemia-induced multipotent stem cell as well as a preparation method and application thereof. The multipotent stem cell is derived from a body cell of a thalassemia patient; and a genome of the thalassemia-induced multipotent stem cell contains a normal beta globin gene. The thalassemia-induced multipotent stem cell disclosed by the invention can be differentiated into hemopoietic stem cells under proper conditions; and the hemopoietic stem cells can express the normal beta globin gene.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Primer pairs and probes for detecting thalassemia genes, kit and use method
InactiveCN112029850AEnhanced fluorescence detection accuracyImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationMedicineGenotype
The invention discloses primer pairs and probes for detecting thalassemia genes. The primer pairs and probes include primer pairs and probes of beta-thalassemia CD41 / 42 and alpha-thalassemia-SEA; theprimer pair of the beta-thalassemia CD41 / 42 is a sequence 1 and a sequence 2, and the probes are a sequence 3 and a sequence 4; and the primer pair of thealpha-thalassemia-SEA is a sequence 5 and a sequence 6 or a sequence 5 and a sequence 7, and the probes are a sequence 8 and a sequence 9. The invention further provides a use method of the primer pairs and probes for detecting the thalassemia genes. The use method comprises the following steps of S1, collecting a sample and extracting DNA; S2, carrying out an amplification reaction by utilizing one kind of primer pair and probes; S3, detecting the fluorescence intensity and determining the genotype; and S4, repeating the process by utilizing the other kind of primer pair and probes. The method has the beneficial effects that the fluorescence detection accuracy is enhanced through the designed primer pairs and probes and the two kinds of primer pairs of the same type; and rapid, accurate and harmless detection is realized by extracting free DNA of plasma and blastocyst culture media of pregnant women.
Owner:成都锦欣生殖医学与遗传学研究所
Gene chip, amplification reagent and kit for detecting alpha-thalassemia
InactiveCN109112200AShort detection timeEasy to operateMicrobiological testing/measurementMolecular diagnostic techniquesThalassemia
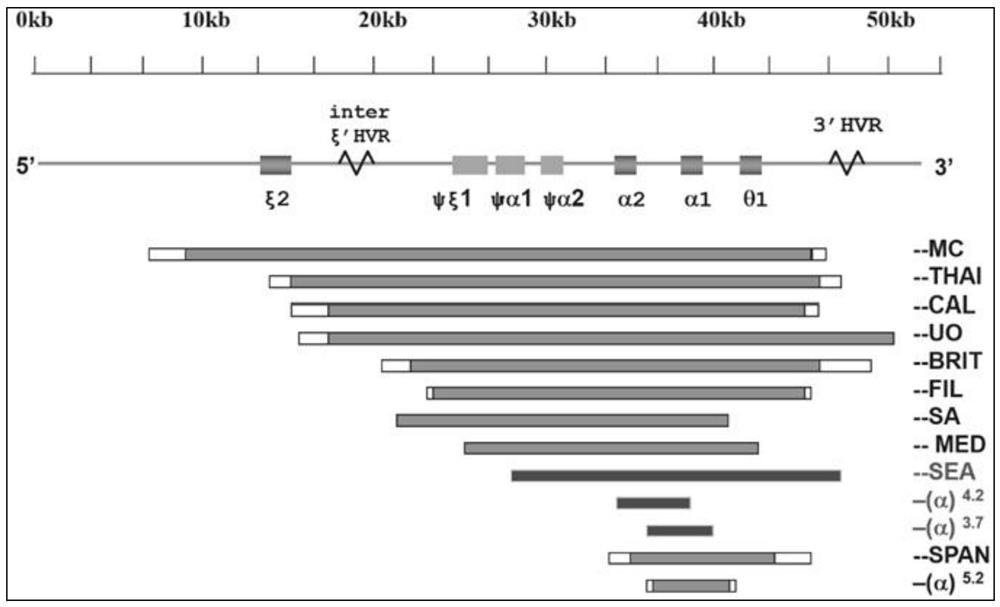
The invention relates to a gene chip, an amplification reagent and a kit for detecting alpha-thalassemia, and belongs to a molecular diagnosis technology, wherein 3 non-deletion (alpha<CS>alpha, alpha<QS>alpha and alpha<WS>alpha) alpha-thalassemia and 4 deletion (--<SEA>, --<THAI>, -alpha<4.2> and -alpha<3.7>) alpha-thalassemia can be rapidly and simultaneously detected by combining direct PCR andreverse dot blot (RDB).
Owner:陈治中
Chip, amplification reagent and kit for directly and simultaneously detecting alpha-thalassemia and beta-thalassemia mutation sites
InactiveCN109112197AFavorable collectionFavorable transportationMicrobiological testing/measurementBeta thalassemiaMolecular diagnostic techniques
The invention relates to a chip, an amplification reagent and a kit for directly and simultaneously detecting alpha-thalassemia and beta-thalassemia mutation sites, and belongs to a molecular diagnosis technology. According to the present invention, based on direct multiplex PCR, Gap-PCR and reverse dot blot combined detection principle, the corresponding amplification primers and the corresponding probes are designed according to the mutation or deletion sites of each genotype, the primer is labeled with biotin, the probe is labeled with amino, a gene chip is used as substrate, the probe is immobilized on the DNA chip, the PCR product amplified by the specific primer is hybridized with the probe, and the diagnosis of thalassemia is performed by interpreting a signal coloring box.
Owner:陈治中
Alpha-thalassemia related gene detection kit
ActiveCN113699231AEasy to operateLow costMicrobiological testing/measurementDNA/RNA fragmentationPrenatal diagnosisNucleotide
The invention discloses an alpha-thalassemia related gene detection kit. The invention provides an alpha-thalassemia detection primer group for detecting alpha globin fusion gene, particularly primers with nucleotide sequences as shown in SEQ ID NO: 2-3, and a detection kit constructed based on the alpha-thalassemia detection primer group; and the operation is simple, the cost is low, the specificity is high, the result interpretation is visual, the popularization is easy, and the alpha-thalassemia detection primer group is suitable for being used in various occasions. Moreover, the primer group also has a very good multi-system general advantage, and can be matched with other detection primers for multiple PCR detection, for example, primers with nucleotide sequences as shown in SEQ ID NO: 4-5 or primers with nucleotide sequences as shown in SEQ ID NO: 6-7 can be used for detecting various types of alpha-thalassemia at one time. The technical scheme provided by the invention is of great significance to screening, genetic counseling and prenatal diagnosis of crowds suffering from thalassemia.
Owner:GUANGZHOU HYBRIBIO MEDICINE TECH LTD +2
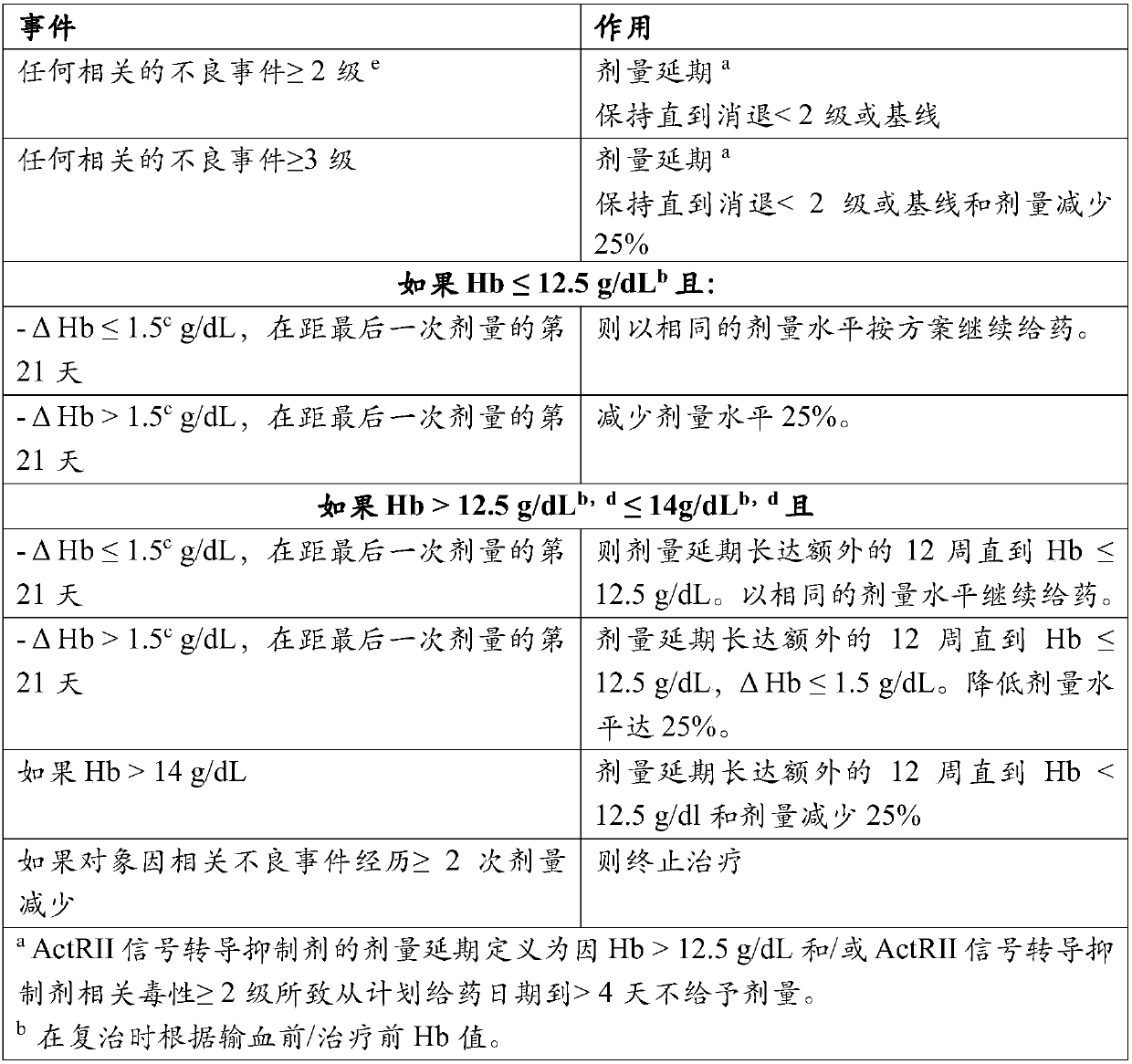
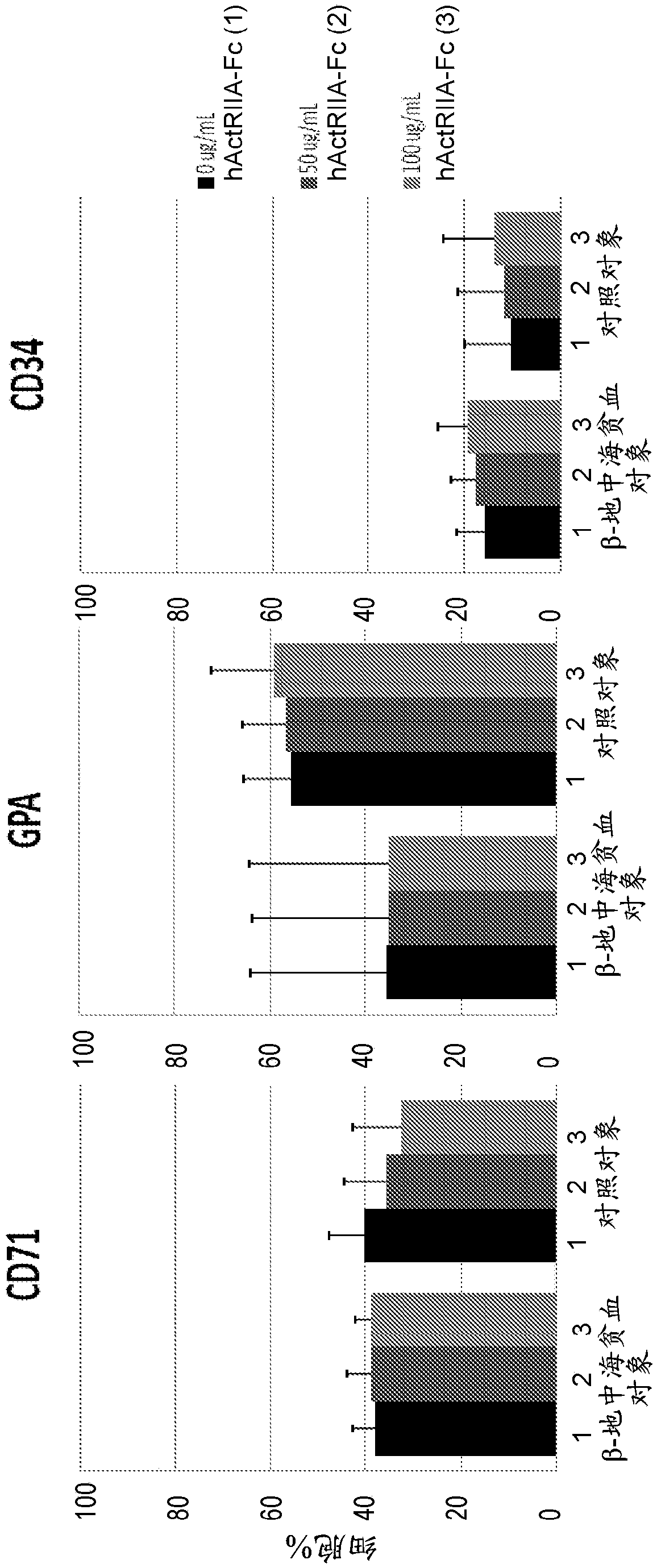
Treatment of beta-thalassemia using actrii ligand traps
PendingCN107847562APeptide/protein ingredientsAntibody mimetics/scaffoldsPharmacologySignal transduction inhibitor
Provided herein are methods of treating beta-thalassemia by subcutaneous administration of about 0.8 mg / kg of an ActRII signaling inhibitor. Also provided herein are methods of adjusting the dose of the ActRII signaling inhibitor administered to the subject.
Owner:CELGENE CORP +1
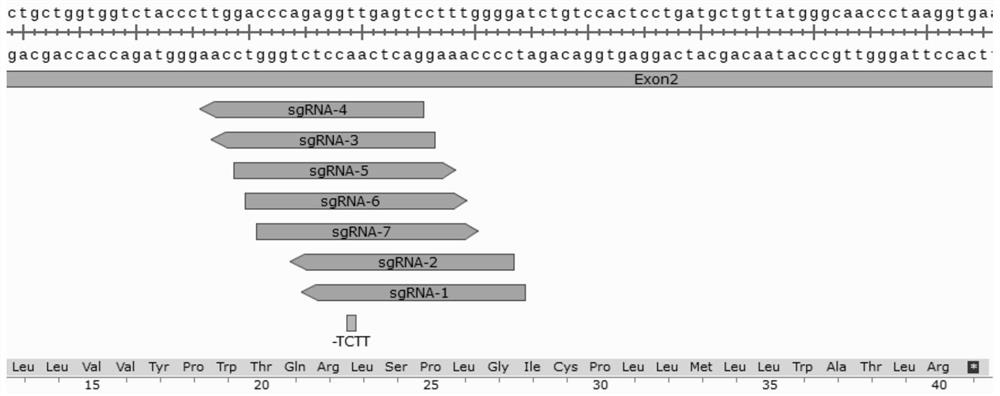
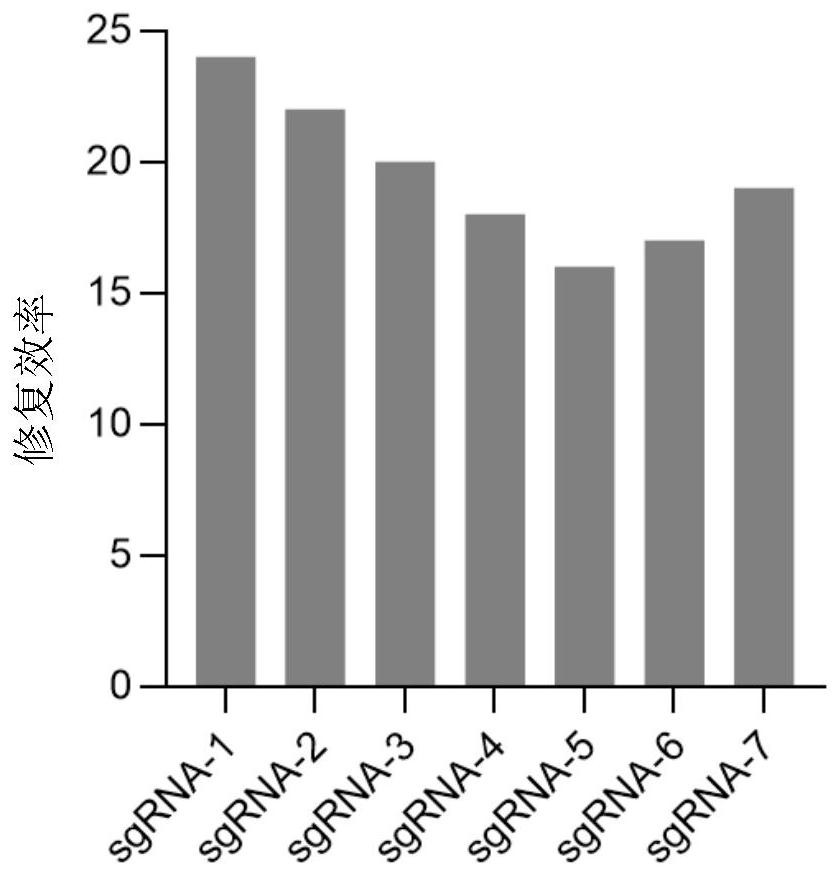
Method and product for repairing HBB gene of hematopoietic stem cell
ActiveCN112746071AEfficient cuttingEfficient modificationHydrolasesStable introduction of DNAAutologous transplantationAmino acid
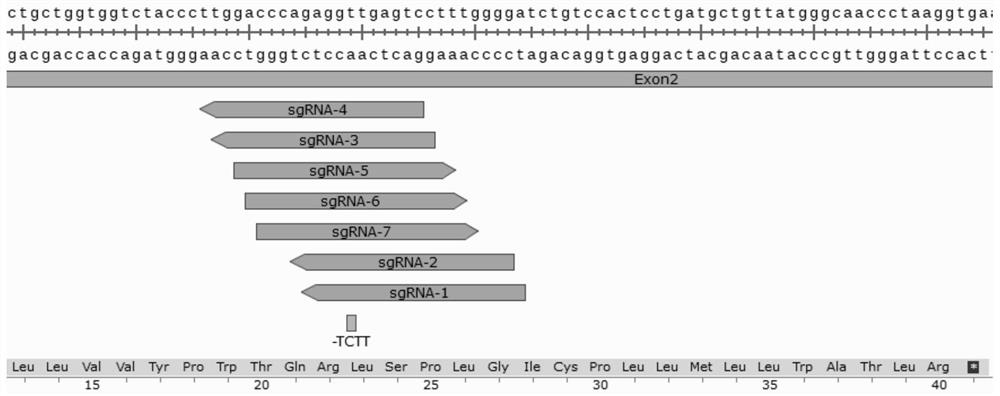
The invention discloses a method and product for repairing the HBB gene of a hematopoietic stem cell. According to the method, the technology that missed site codon 41 / 42(-TCTT) in beta-thalassemia (thalassemia) is subjected to targeted knockout by utilizing a CRISPR-Cas9 gene editing technology; sgRNA capable of identifying and guiding Cas9 protein to the target sequence of a target gene is designed and synthesized; and the sgRNA and Cas9 protein are mixed and electrically transfected into the hematopoietic stem cell of the beta-thalassemia codon 41 / 42 (-TCTT), meanwhile, a homologous recombination donor is introduced to efficiently repair the normal coding function of amino acid at the mutation site and recover the normal expression of the beta-thalassemia gene. The transfusion dependent type beta-thalassemia codon 41 / 42 (-TCTT) is edited by utilizing the existing gene editing technology which has high repairing efficiency, the repaired hematopoietic stem cells of the patient can reconstruct the blood system of the patient and treat thalassemia after autotransplantation.
Owner:EAST CHINA NORMAL UNIV +1
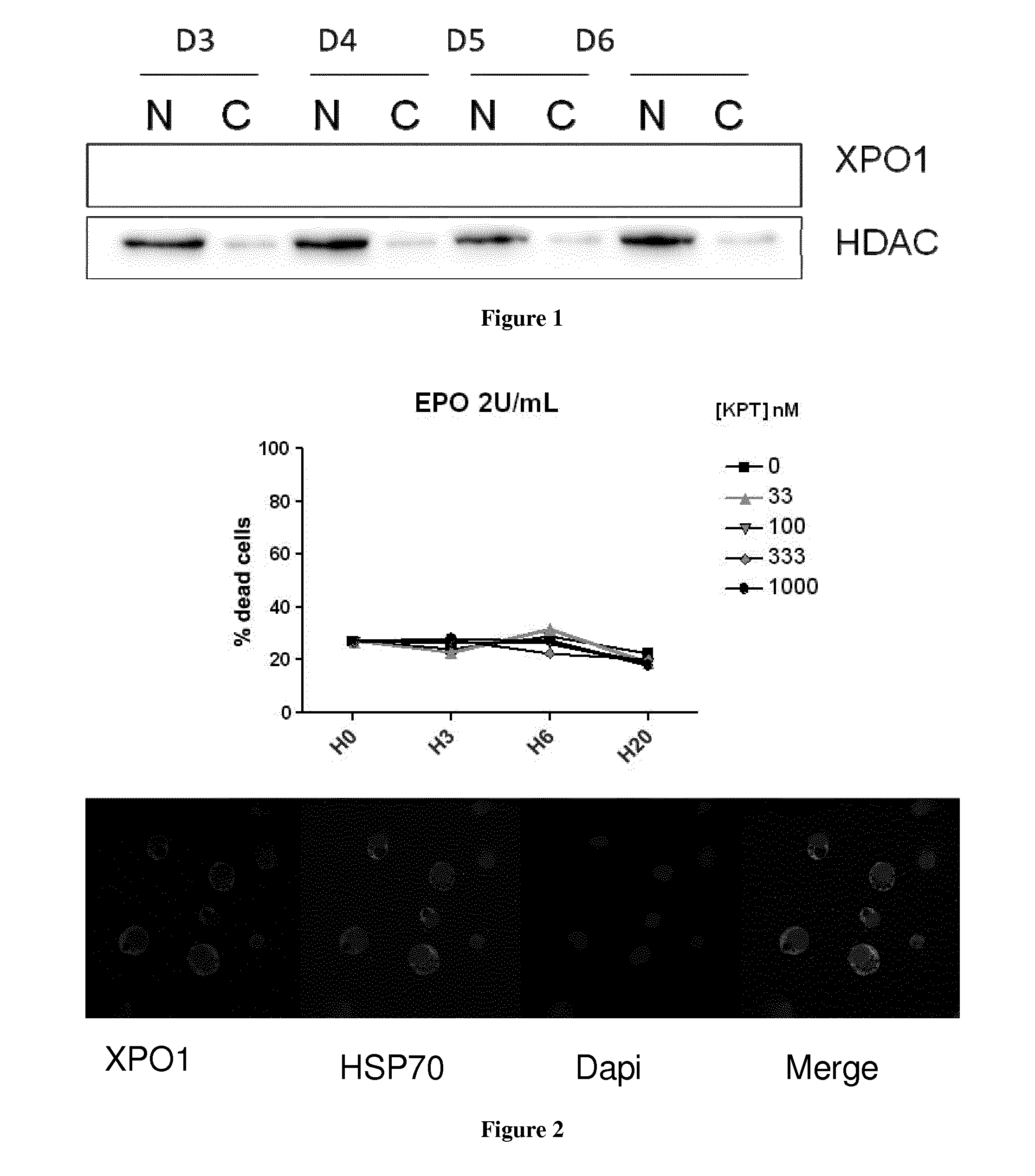
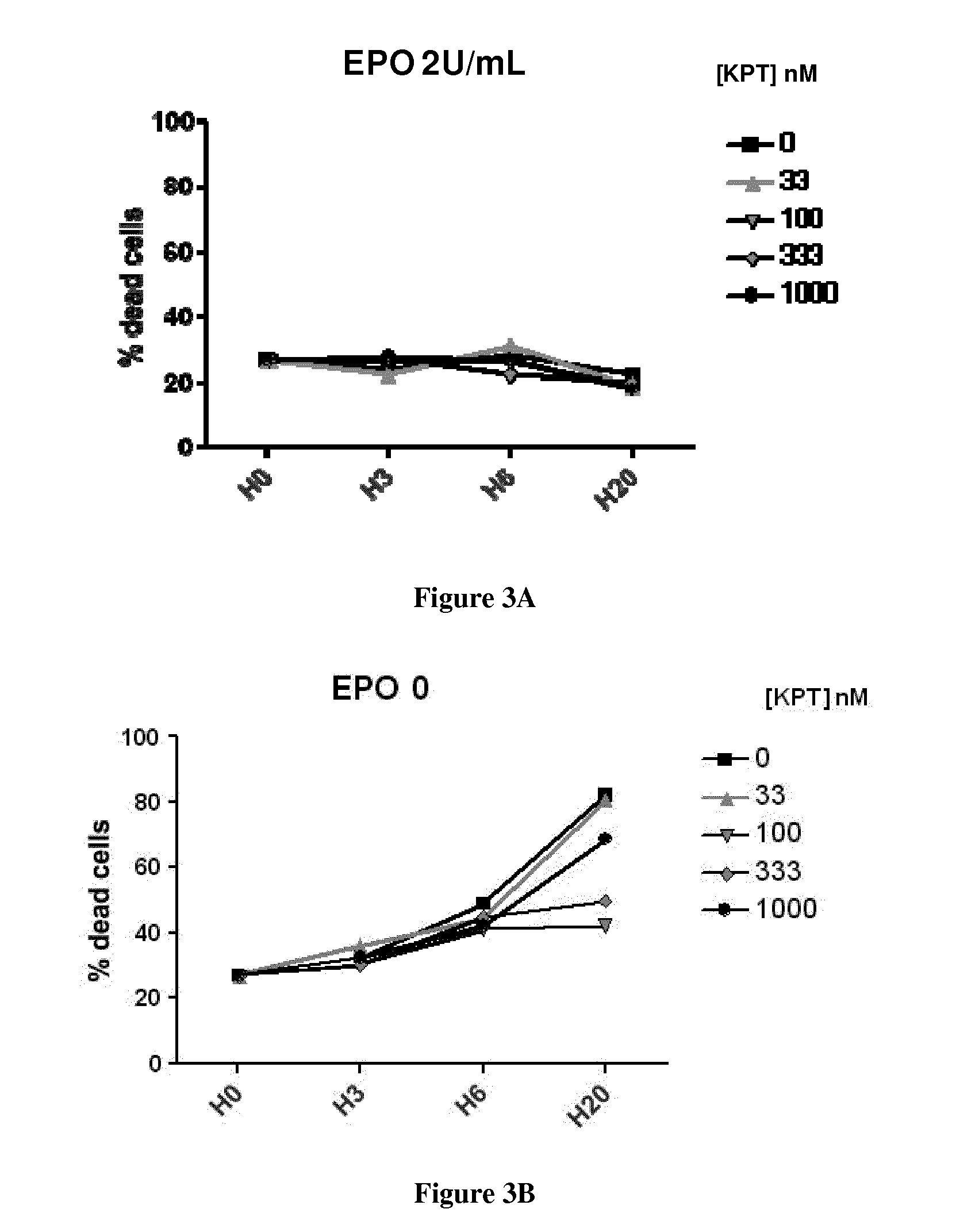
Methods and pharmaceutical compositions for the treatment of beta-thalassemias
InactiveUS20170020847A1Extended half-lifeImprove stabilityCompound screeningApoptosis detectionPharmaceutical drugPharmacology
The present invention relates to methods and pharmaceutical compositions for the treatment of beta-thalassemias. In particular, the present invention relates to an XPO1 inhibitor for use in a method for treating beta-thalassemia in a subject in need thereof.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
Alpha-thalassemia screening kit and application thereof in prenatal screening
ActiveCN103421903BReasonable primer designAchieving Prenatal ScreeningMicrobiological testing/measurementPrenatal screeningBiology
The invention discloses an α-thalassemia screening kit and its application in prenatal screening, belonging to the technical field of α-thalassemia prenatal screening. The α-thalassemia screening kit includes: (1) Negative control sample; (2) Positive control sample; (3) PCRMasterMIX; (4) PCR primers. Its application uses designed primers to amplify fetal DNA in the peripheral blood of pregnant women to achieve prenatal screening for α-thalassemia. The present invention collects the peripheral blood of pregnant women for prenatal screening of α-thalassemia, without puncturing the amniotic cavity and inserting villi tissue, without any trauma to the fetus, and is safe and reliable; the accuracy rate can reach 99.99%, filling the non-invasiveness of α-thalassemia The technical gap in prenatal screening reduces the birth rate of sick children.
Owner:邯郸市康业生物科技有限公司
Primer set for enriching thalassemia genes through long-fragment PCR and application thereof
ActiveCN112342289AEasy to detectGood amplification effectMicrobiological testing/measurementDNA/RNA fragmentationGeneVirology
The invention discloses a primer set for enriching thalassemia genes through long-fragment PCR and application thereof. The inventor obtained experimental data of optimized primer set for enriching the thalassemia gene by long-fragment PCR through a self-owned technology, which shows that the primer has a very good amplification effect, and the required long-fragment gene can be efficiently amplified, and the detection of the thalassemia is greatly simplified.
Owner:GUANGZHOU JINGKE DX CO LTD +1
Application of thyroid hormone and analogue thereof in preparation of drug for treating alpha-thalassemia
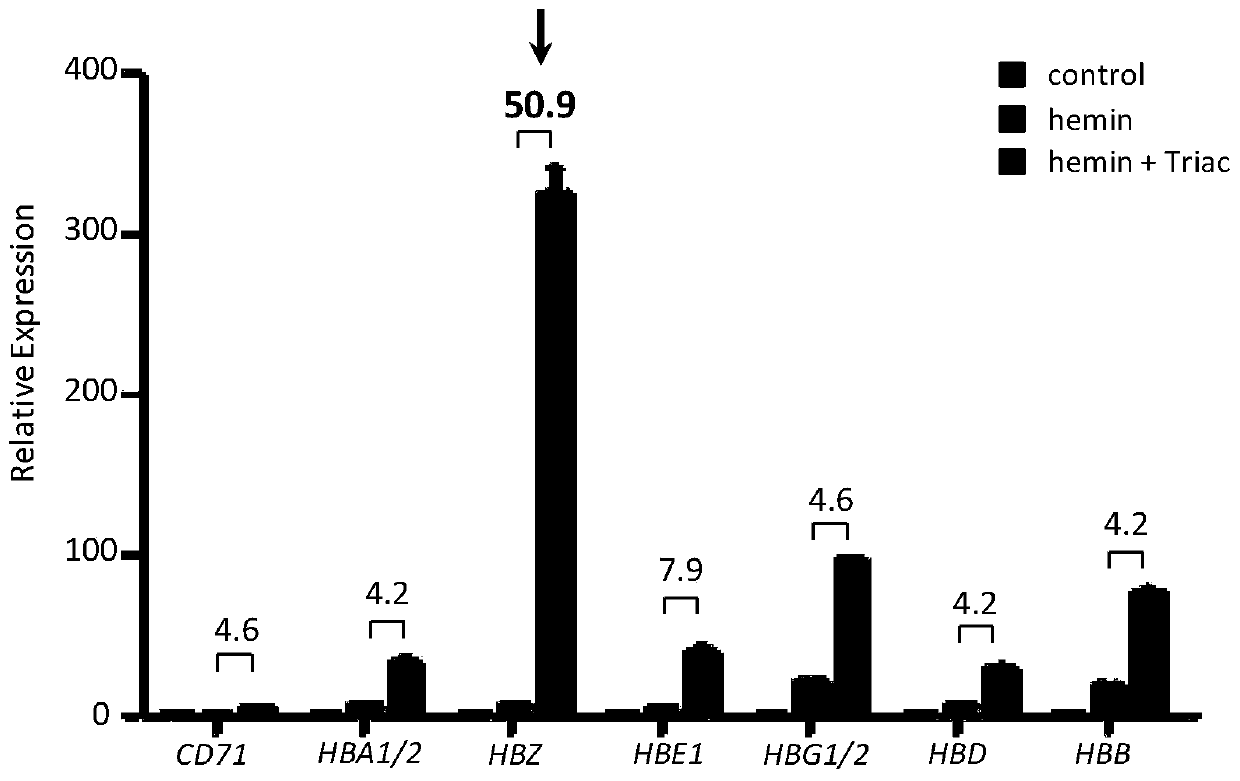
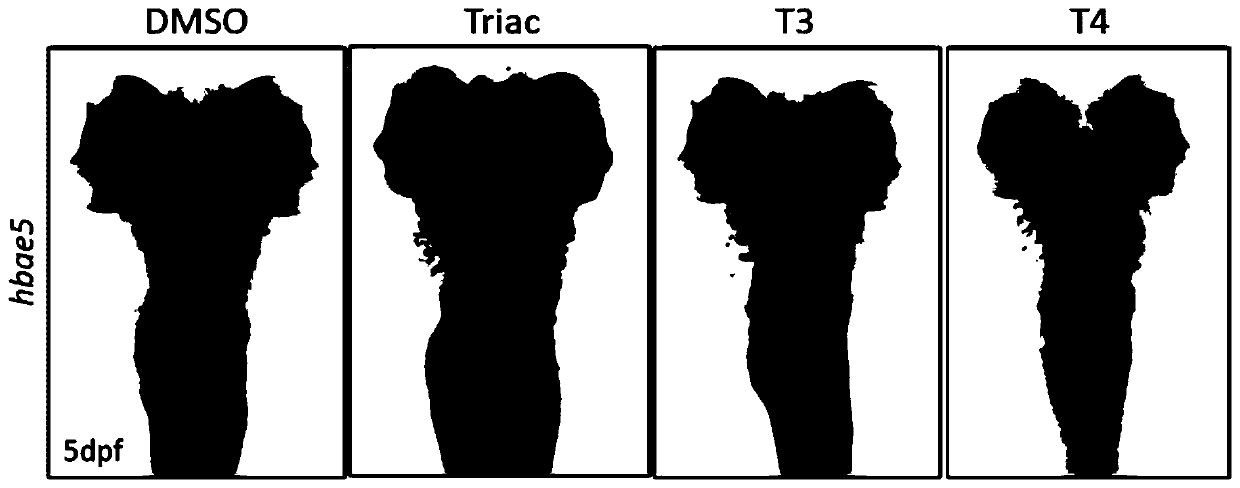
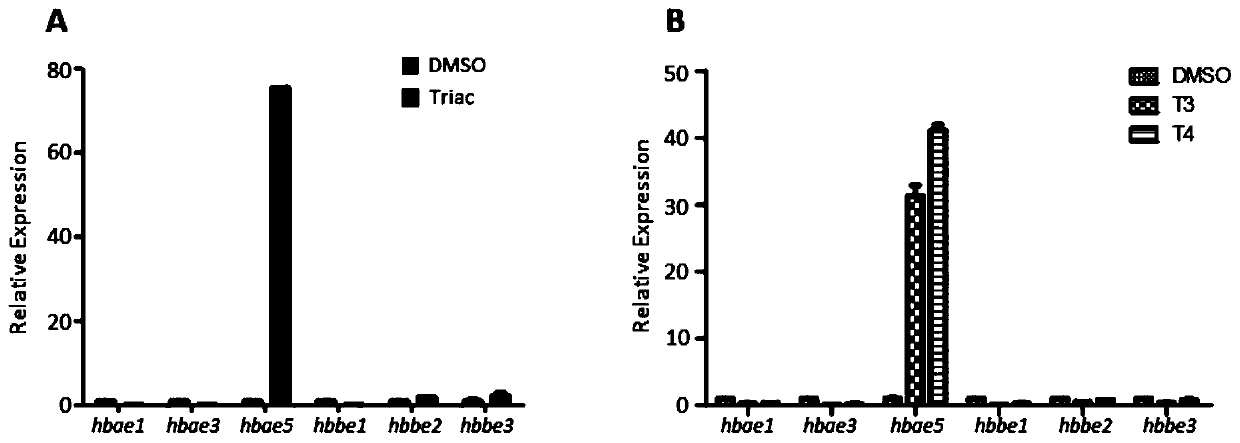
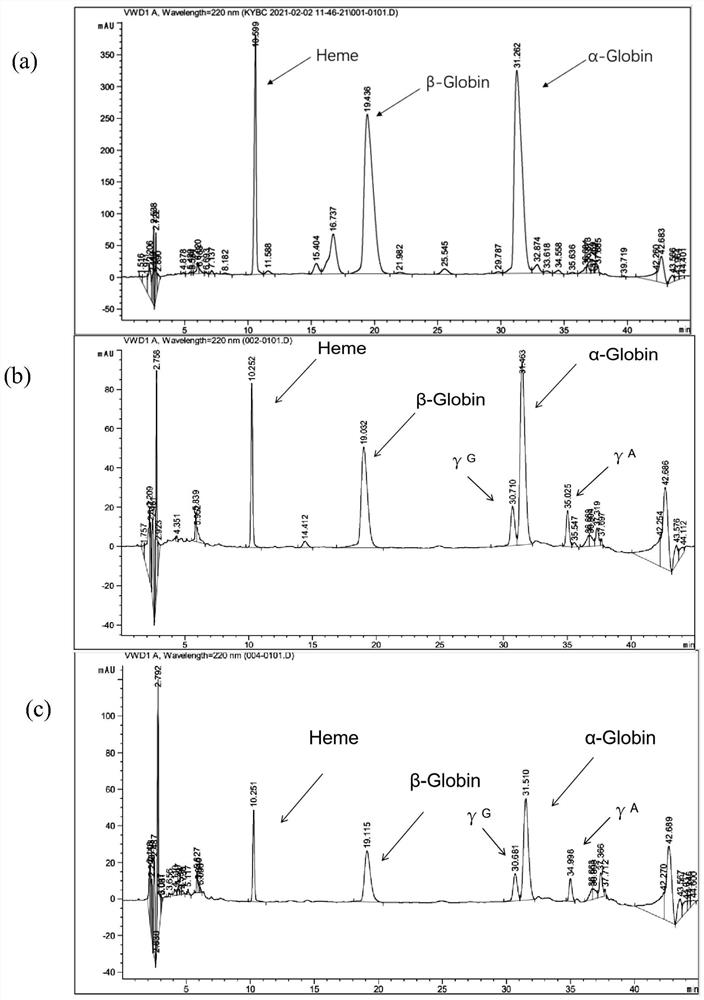
The invention provides an application of thyroid hormone and an analogue thereof in preparation of a drug for treating alpha-thalassemia. The thyroid hormone and the analogue can be specifically applied in regulation of expression of zeta-globin genes, in the differentiation process of K562 cells, the thyroid hormone analogue can be specifically and significantly up-regulate the expression of thezeta-globin gene (HBZ) by up to 50 times, and in model animal zebrafish embryos, after treatment is performed by using the thyroid hormone and the analogue, the expression of the zeta-globin gene (hbae5) can also be specifically up-regulated by up to 30-70 times; therefore, the thyroid hormone and the analogue thereof can be used to specifically activate the expression of the zeta-globin genes, that is, to reactivate the silent zeta-globin genes in patients with the alpha-thalassemia to inhibit destruction of red blood cells, the thyroid hormone and the analogue realizes a method for preparingthe drug for treating the alpha-thalassemia, the economical, safe and effective method is provided for treatment of the alpha-thalassemia, and the method can be widely promoted and used.
Owner:SHANGHAI SPH RARE DISEASE PHARMA CO LTD
Lentivirus carrying erythroid gene editing system and medicine
ActiveCN113046330AIncreased erythroid specificityStrong infection abilityBlood/immune system cellsNon-active genetic ingredientsLentivirusElectroporation
The invention relates to a lentivirus carrying an erythroid gene editing system. The lentivirus carries a sequence of a CRISPR-Cas9 gene editing system of a specific targeting BCL11A enhancer. According to the invention, the CRISPR-Cas9 gene editing system of the specific targeting BCL11A enhancer is delivered into hematopoietic stem cells through a lentiviral vector, BCL11A is inhibited in a targeting manner, and gamma-globin expression is reactivated, so that beta-thalassemia is relieved or cured. The lentiviral vector is used as a delivery system, and compared with an electroporation method commonly adopted at home and abroad, the lentiviral vector has the advantages of being high in infection capacity, easy to operate, high in efficiency and high in safety.
Owner:GENMEDICN BIOPHARMA INC
Primer and probe combination and kit for multiplex real-time fluorescence PCR detection of beta-thalassemia gene mutation
ActiveCN111593115AImprove throughputAchieving Specific DetectionMicrobiological testing/measurementAgainst vector-borne diseasesSpecific detectionWild type
The invention provides a primer and probe combination and a kit for multiplex real-time fluorescence PCR detection of beta-thalassemia gene mutation, and belongs to the technical field of molecular biology detection. The primer and probe combination comprises one or more of a first group of primer and probe combination to a twelfth group of primer and probe combination; each group of primer and probe combination comprises primers and probes for detecting two beta-thalassemia gene mutation types and two corresponding wild types; and conditions of the mutation types are indicated by an FAM fluorescence channel or an HEX fluorescence channel, and the wild types are indicated by an ROX fluorescence channel or a CY5 fluorescence channel. The primer and probe combination can simultaneously detect two or more sites in the mutation types and the wild types of a beta-thalassemia mutant gene, and reads information and a detection result through four different fluorescence channels, so that high-throughput and specific detection of beta-thalassemia is achieved.
Owner:厦门安普利生物工程有限公司 +1
A method and product for repairing hbb gene of hematopoietic stem cells
ActiveCN112746071BEfficient cuttingEfficient modificationHydrolasesStable introduction of DNAGlobin genesAutologous transplantation
The invention discloses a method and product for hematopoietic stem cell HBB gene repair. The method utilizes CRISPR-Cas9 gene editing technology to target knockout of codon 41 / 42(-TCTT) in β-thalassemia (thalassemia) ) technology, by designing and synthesizing sgRNA that can recognize and guide Cas9 protein to the target sequence of the target gene, mix it with Cas9 protein and electrotransduce it into β-thalassaemia codon 41 / 42 (‑TCTT) hematopoietic stem cells, and introduce homologous The recombinant donor efficiently restores the normal coding function of the amino acid at the mutation site and restores the normal expression of the β-globin gene. The present invention utilizes the existing gene editing technology to edit transfusion-dependent β-thalassemia codon 41 / 42 (-TCTT), which has high repair efficiency, and the repaired patient's hematopoietic stem cells can rebuild the patient's blood system and treat thalassemia after autologous transplantation .
Owner:EAST CHINA NORMAL UNIV +1
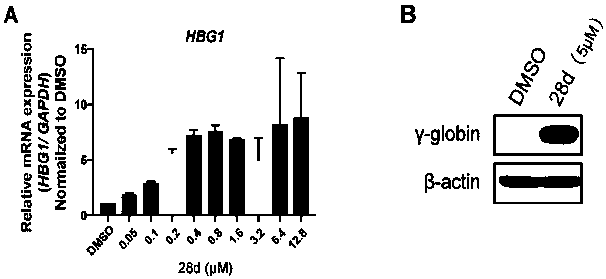
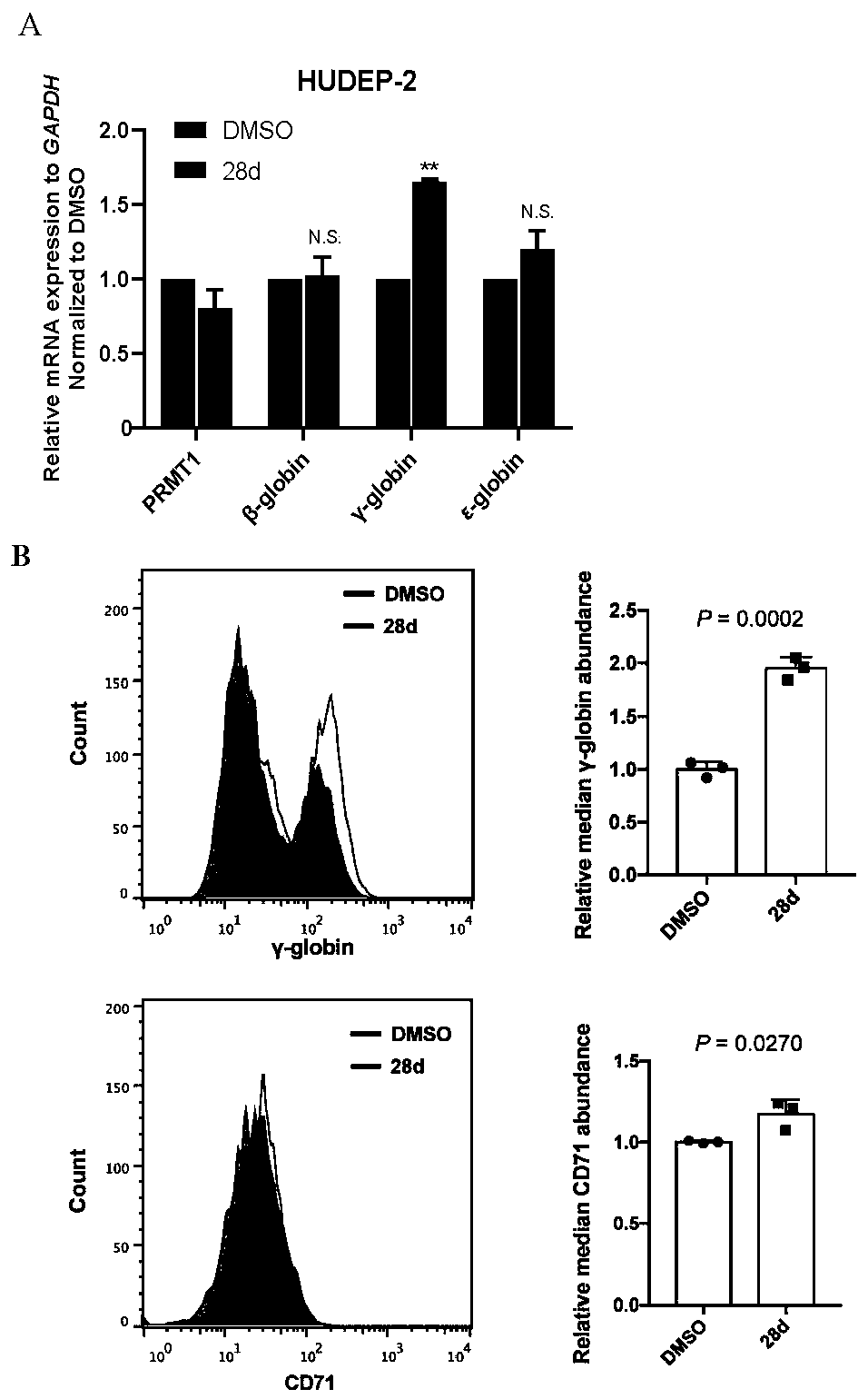
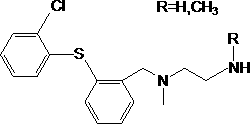
Application of compound 28d in preparation of medicine for increasing expression quantity of gamma-globin
ActiveCN111481532ALow action concentrationImprove expression levelOrganic active ingredientsBlood disorderCoboglobinChemical compound
Owner:CHINA AUSTRALIA INST OF TRANSLATIONAL MEDICINE CO LTD NANJING CHINA
Chip and kit for detecting non-deletion alpha-thalassemia
InactiveCN109112183AReduce pollutionSave manpower and reagent costsMicrobiological testing/measurementBiotinDna microchips
The invention relates to a gene chip, a PCR amplification reagent and a kit for detecting non-deletion alpha-thalassemia genes, and belongs to a molecular diagnosis technology. According to the present invention, based on direct multiplex PCR, Gap-PCR and reverse dot blot combined detection principle, the corresponding amplification primers and the corresponding probes are designed according to the mutation or deletion sites of each genotype, the primer is labeled with biotin, the probe is labeled with amino, a gene chip is used as substrate, the probe is immobilized on the DNA chip, the PCR product amplified by the specific primer is hybridized with the probe, and the diagnosis of thalassemia is performed by interpreting a signal coloring box.
Owner:陈治中
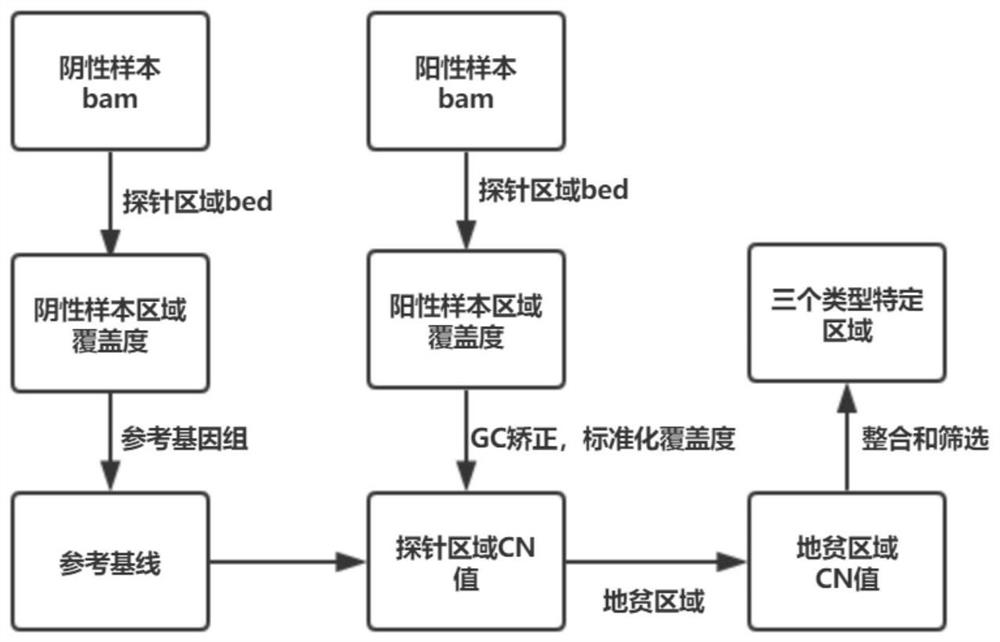
Detection probe, method, chip and application of α and/or β-thalassemia mutation based on whole gene capture sequencing
ActiveCN106591441BEnables detection of deletions in large regionsMicrobiological testing/measurementDNA/RNA fragmentationChromosome regionsNew mutation
The present invention provides a detection primer, method, chip and application of α and / or β-thalassemia point mutations and deletion mutations based on whole gene capture sequencing. The present invention enriches all related genes involved in α and β thalassemia by designing capture probes, detects mutation information such as all SNPs and indels in the full-length sequences of each gene, and simultaneously increases The upstream and downstream regions of each coding gene are used as a reference to detect structural variations such as SNVs and CNVs. Compared with the current detection techniques of various hotspot mutation sites, the present invention can not only detect the information of hotspot mutations, but also detect some rare mutations and undiscovered new mutation types, and realize the specific detection and analysis of the full-length sequence of the target gene. Full coverage of types, greatly making up for the missed detection of low-frequency mutations and rare mutations by conventional detection methods.
Owner:高飞 +1
Deletion-type alpha-thalassemia detection kit based on Taqman probe and detection method of deletion-type alpha-thalassemia detection kit
ActiveCN112553318AReduced amplification efficiencyAmplified and detectedMicrobiological testing/measurementDNA/RNA fragmentationWild typeStain
The invention provides a multiplicity multicolor real-time fluorescence PCR method and a detection kit regarding the defects of existing deletion-type alpha-thalassemia detection methods and detectionsites. By means of the method, deletion-type alpha-thalassemia (such as --SEA, -alpha 3.7, -alpha 4.2 and -THAI deletion which are common in China) can be detected, whether sites exist or not is determined by detecting genotypes, whether alleles of alpha-thalassemia of a to-be-examined person are wild type alleles, heterozygous-deletion-type alleles or homozygous-deletion-type alleles is judged,and thus the method is beneficial to clinical genetic counseling. Meanwhile, the method provided by the invention can effectively amplify long fragments with high GC content, and provides a real-timefluorescence PCR detection method and an amplification system suitable for the Taqman probe in combination with an optimized reaction program and reaction system. The method has the advantages of simplicity, convenience, stain resistance, high sensitivity, stability and accuracy, high specificity and the like, the time for clinical thalassemia screening and diagnosis can be greatly shortened, andthe clinical efficiency is improved.
Owner:CARRIER GENE TECH SUZHOU CO LTD
Correction of Beta-Thalassemia Phenotype by Genetically Engineered Hematopoietic Stem Cell
PendingUS20220184137A1The process is simple and fastHaemoglobins/myoglobinsGenetically modified cellsCoboglobinGlobin genes
The present invention relates to a genetically modified hematopoietic stem cell (HSC) comprising, in at least one α-globin gene comprised in the genome thereof, at least one transgene encoding a functional β-like globin protein, the said transgene being placed under the control of the endogenous promoter of the said at least one α-globin gene.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Application of thalidomide to preparation of pharmaceutical composition for improving liver function damage of patients with thalassemia
ActiveCN111374978AProtection from damageTo promote metabolismOrganic active ingredientsDigestive systemLiver functionsSerum glutamate pyruvate transaminase
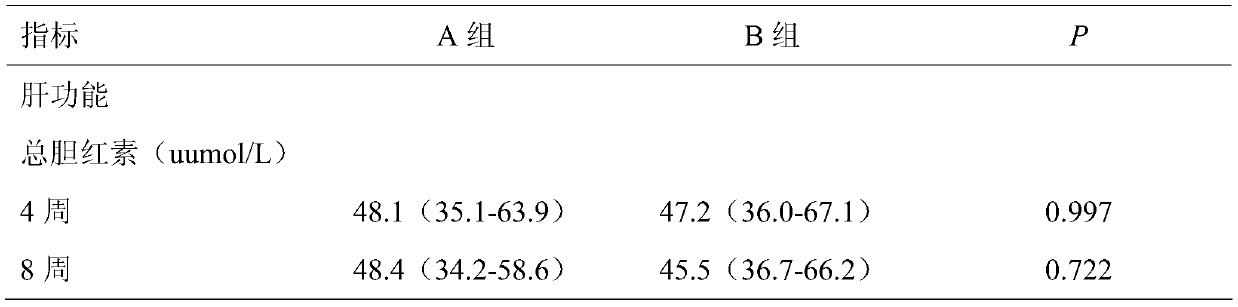
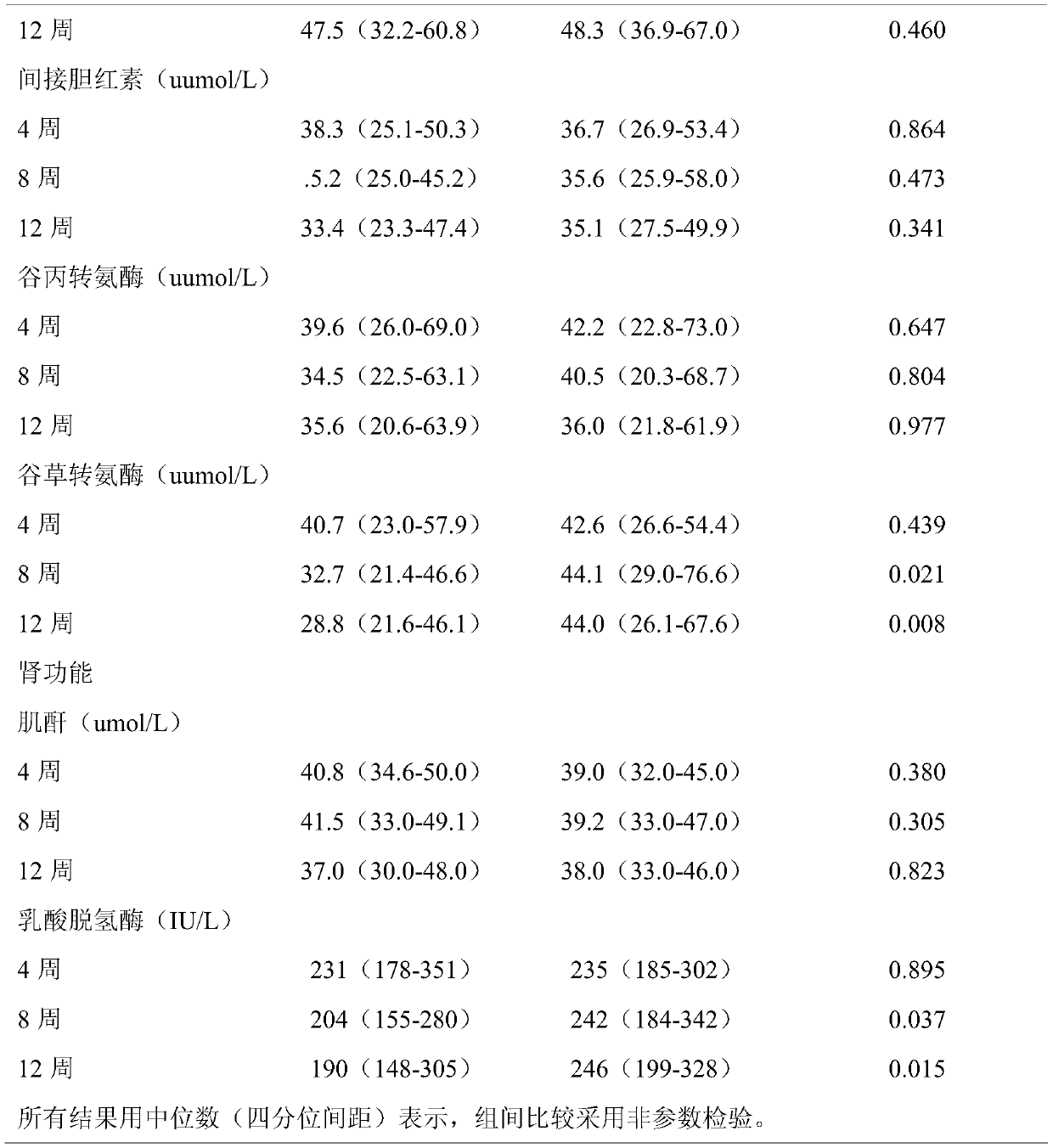
The invention discloses application of thalidomide to preparation of a pharmaceutical composition for improving liver function damage of patients with thalassemia. Biochemical indexes of patients during treatment show that the aspartate aminotransferase (AST) level representing a liver function in a thalidomide group is significantly reduced after 8 weeks of treatment by monitoring liver functiontests of the patients during a follow-up visit; the AST level in a placebo group is significantly increased, and the AST level of the thalidomide group is significantly lower than that of the placebogroup (32.7 VS 44.1, P=0.021). By 12 weeks, the advantage of reduction of the AST level in the thalidomide group is further expanded (28.8 VS 44.0, P=0.008), and it is indicated that the thalidomide can effectively improve the liver function levels of the patients with thalassemia, and suggests that the thalidomide has a good therapeutic effect on liver function damage of the patients with the thalassemia.
Owner:梧州市工人医院
PCR reagent and kit for detecting beta-thalassemia
InactiveCN111593112ASave time and costFast detection timeMicrobiological testing/measurementDNA extractionWhole blood sample
The invention provides a PCR reagent and kit for detecting beta-thalassemia. According to the PCR reagent, a whole blood sample for beta-thalassemia detection is amplified by adopting a high-tolerancedirect spreading PCR enzyme; and a genome DNA in the sample does not need to be extracted. According to the detection kit obtained by using the PCR reagent, the whole detection time of detection personnel is shortened, and the time cost is greatly saved; and meanwhile, DNA extraction and purification processes are reduced, so that the factors of influencing the detection accuracy are reduced, andthe accuracy of the detection result is greatly improved.
Owner:深圳市星蝶科技有限公司
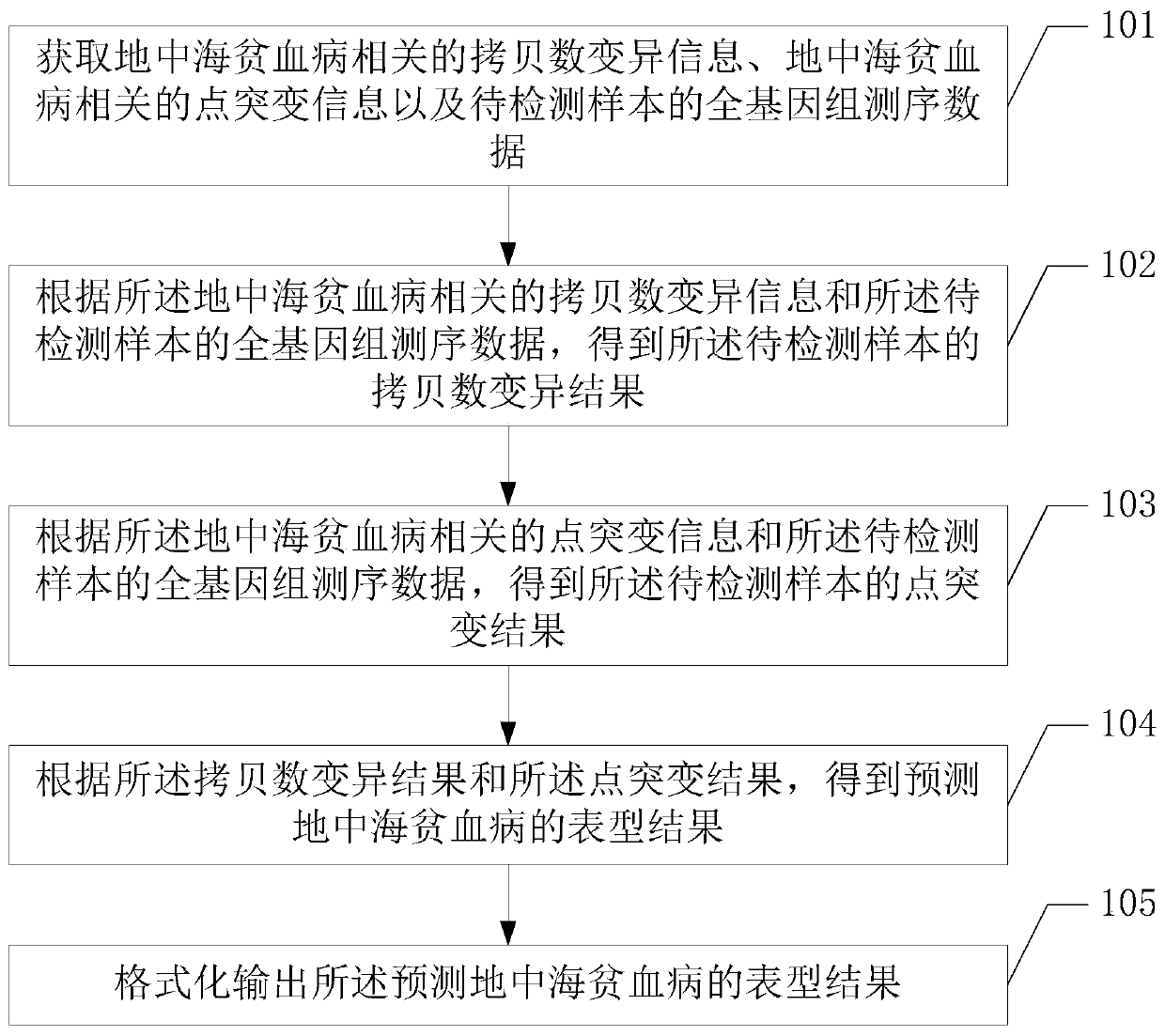
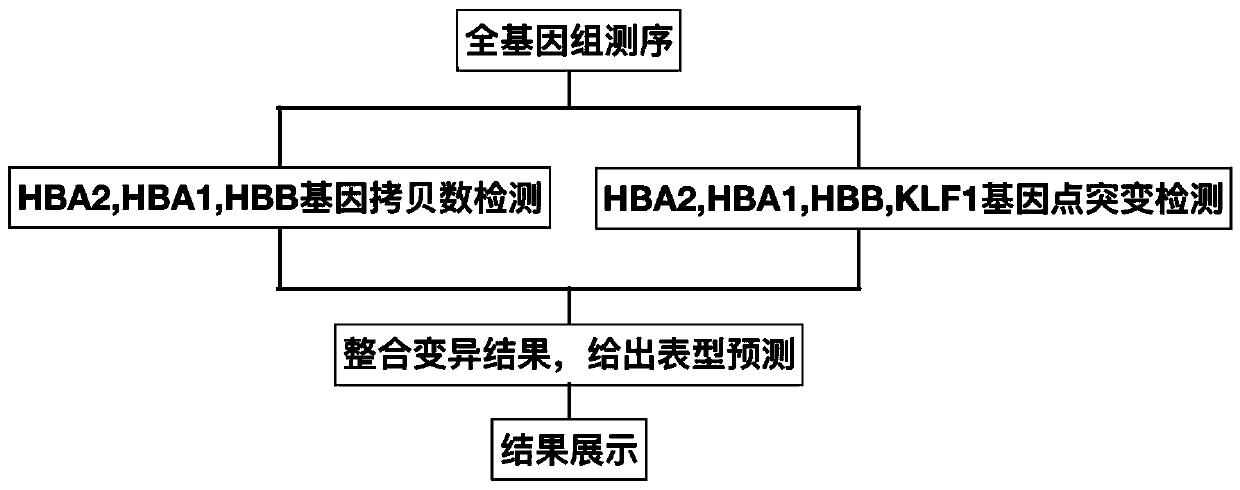
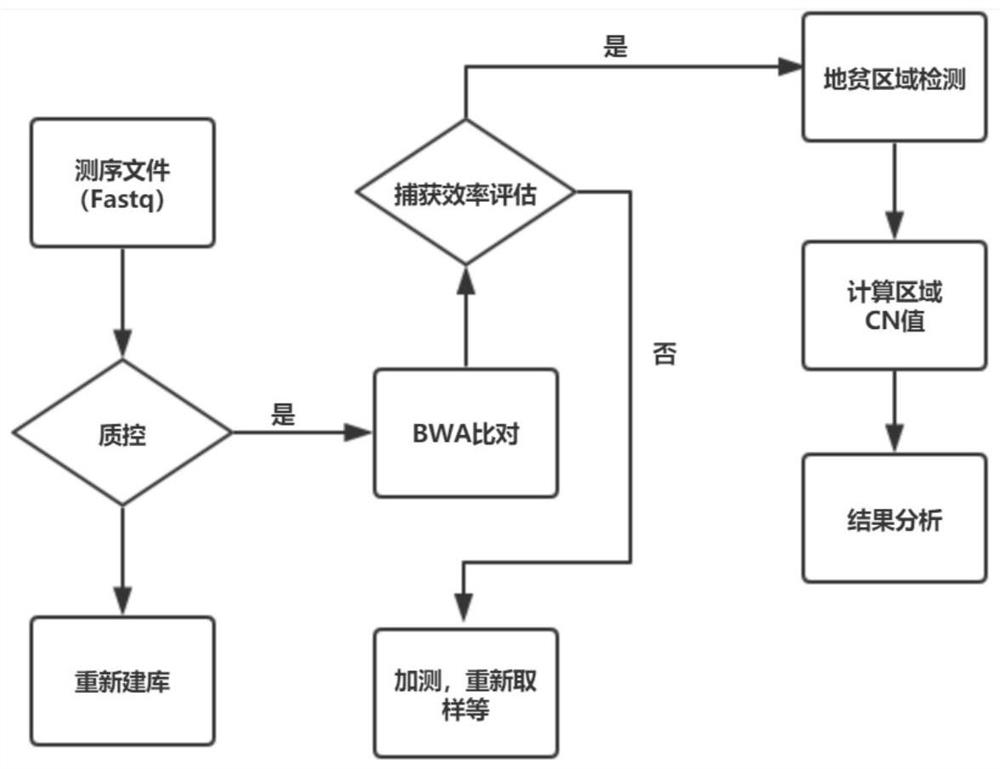
Method and device for detecting thalassemia gene variation
The embodiment of the invention discloses a method and a device for detecting thalassemia gene variation and a computer readable storage medium, which are used for detecting most variation related tothalassemia by utilizing one-time sequencing and predicting a phenotypic result of an individual thalassemia disease according to a variation detection result. The method provided by the embodiment ofthe invention comprises the following steps: acquiring copy number variation information related to thalassemia, point mutation information related to thalassemia and whole genome sequencing data ofa to-be-detected sample; obtaining a copy number variation result of the to-be-detected sample according to the copy number variation information related to thalassemia and the whole genome sequencingdata of the to-be-detected sample; obtaining a point mutation result of the to-be-detected sample according to the point mutation information related to the thalassemia disease and the whole genome sequencing data of the to-be-detected sample; and obtaining a phenotypic result for predicting thalassemia according to the copy number variation result and the point mutation result.
Owner:深圳市早知道科技有限公司
In vitro cell culture methods for beta-thalassemia using activin type ii receptor ligand traps
PendingCN108350057APeptide/protein ingredientsAntibody mimetics/scaffoldsCell biologyIn vitro cell culture
Provided herein are methods of treating beta-thalassemia in a subject comprising administering to the subject an activin type II receptor (ActRII) signaling inhibitor (e.g., an activin ligand trap) and utilizing one or more in vitro cell culture methods provided herein in (i) selection of the subject to be treated according to the methods provided herein; and / or (ii) monitoring of the subject being treated according to the methods provided herein.
Owner:CELGENE CORP +1
CRISPR/Cas9 systems and application of CRISPR/Cas9 systems in construction of alpha, beta and alpha-beta thalassemia model pig cell lines
ActiveCN112538497AImprove editing efficiencyKnockout EfficientHydrolasesGenetically modified cellsGenome editingFibroblastic cell
The invention discloses CRISPR / Cas9 systems and an application of the CRISPR / Cas9 systems in construction of alpha, beta and alpha-beta thalassemia model pig cell lines. A pair of target sequences isdesigned for each of pig HBA and HBB genes, three CRISPR / Cas9 systems are constructed by the two pairs of target sequences, each CRISPR / Cas9 system comprises a gRNA carrier and a Cas9 expression carrier, the expression carriers in the three CRISPR / Cas9 systems are respectively transferred into pig fibroblasts in proportion, and the alpha, beta and alpha-beta thalassemia model pig cell lines are obtained by screening. Single gene mutation and combined gene mutation are respectively caused to the pig HBA and HBB genes in the pig primary fibroblasts through a gene editing technology, and HBA, HBBand HBA-HBB gene mutant cells are obtained.
Owner:NANJING KGENE GENETIC ENG CO LTD
Gene detection kit for Hong Kong alpha-thalassemia
The invention relates to the technical field of biology and medicament, and particularly relates to a gene detection kit for Hong Kong alpha-thalassemia. According to the invention, the gene sequence of HK alpha alpha fusion gene is further confirmed and compared with alpha-globin sequence for analysis, and primer design is carried out in a conserved sequence area of the fusion gene. The technical scheme of the invention is to provide a gene detection kit for Hong Kong alpha-thalassemia, comprising a PCR (polymerase chain reaction) solution, wherein the PCR solution contains primer HK alpha alpha-F and HK alpha alpha-R, the primer is designed aiming at the conserved sequence area of the HK alpha alpha fusion gene, and the nucleotide sequence of the conserved sequence of the HK alpha alpha fusion gene is shown in SEQ ID NO:1. The kit disclosed by the invention can be directly used for kits for HK alpha alpha detection and can specifically, quickly and stably diagnose HK alpha alpha gene type.
Owner:亚能生物技术(深圳)有限公司
Primer, method and reagent kit for detecting HBB gene mutation
PendingCN111733232ALearn about mutationsEasy to operateMicrobiological testing/measurementDNA/RNA fragmentationMedicineExon
The invention discloses a method, primer and reagent kit for detecting HBB gene mutation pertinent to beta thalassaemia. The primer and reagent kit both comprise an amplifying primer for HBB gene whole exons and a sequencing primer. Based on PCR amplification and Sanger sequencing, the mutation situation of an HBB gene mutation site pertinent to the beta thalassaemia can be quickly detected.
Owner:南京艾迪康医学检验所有限公司
PCR amplification primer set, amplification reagent and kit for rapidly detecting Thailand alpha-thalassemia
InactiveCN109112196AImprove stabilityIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationMolecular diagnostic techniquesThalassemia
The invention relates to a PCR amplification primer set, an amplification reagent and a kit for rapidly detecting a Thailand alpha-thalassemia, and belongs to a molecular diagnosis technology. According to the present invention, the application of the detection kit of the present invention to detect --<THAI>-deletion thalassemia only consumes 2 h; the detection result of the detection kit of the present invention is consistent with the detection result of the conventional Gap-PCR method using DNA extraction, wherein the conventional Gap-PCR method using DNA extraction requires more than 4 h, and further has disadvantages of high cost, cumbersome operation and easy contamination and degradation of DNA while the detection of --<THAI>-deletion thalassemia with the Direct PCR method has advantages of rapidness, sensitivity, safety and the like, such that the kit of the present invention can be used for detecting --<THAI> deletion thalassemia.
Owner:陈治中
Capture probe composition of three subtypes of thalassemia and its application method and application device
ActiveCN110592208BEasy constructionMicrobiological testing/measurementSequence analysisBioinformaticsReference genome
The invention provides a capture probe composition for three subtypes of thalassemia, an application method and an application device. Wherein, the preparation method of the capture probe composition of the three subtypes of thalassemia includes: separating the full-length regions of the genes related to the three subtypes of thalassemia into different small fragments according to preset lengths; The fragments are compared to the human reference genome to obtain the first single alignment region set and multiple alignment region sets; move the multi-alignment region set forward or backward on the human reference genome by a length of kbp, k≤100, and move The final new region is re-aligned with the human reference genome to obtain a second single alignment region set; probe design is performed from the first single alignment region set and the second single alignment region set to obtain a capture probe composition. The capture probe composition obtained by the method can not only mark the whole region but also can distinguish three different thalassemia types, so that the detection is more accurate.
Owner:BEIJING NOVOGENE TECH CO LTD +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com