Application of thalidomide to preparation of pharmaceutical composition for improving liver function damage of patients with thalassemia
A technology for thalassemia and thalidomide, applied in pharmaceutical compositions, the field of thalidomide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of capsules:
[0032] Thalidomide is provided by Changzhou Pharmaceutical Factory of Shanghai Pharmaceutical Group, and the preparation method of Thalidomide capsules is as follows:
[0033] 1) Take 100g of thalidomide, micronize it, and pass through a 200-mesh sieve to obtain thalidomide fine powder;
[0034] 2) Put 50g of poloxamer 237 in a suitable container, put it in a water bath at 49°C-50°C and heat it to a molten liquid state, add 100g of thalidomide fine powder, stir quickly and fully at 49°C-50°C until mixed Evenly, let it stand to remove air bubbles, spread the mixture into a thin layer and put it in a -5°C refrigerator for rapid cooling. After the mixture is completely solidified, take it out and pulverize it, and dry it in a vacuum dryer at 35°C for 24 hours, and pulverize it through a 100-mesh sieve to obtain Thalidomide solid dispersion;
[0035] 3) Add 82g of microcrystalline cellulose, 10g of lactose, 8g of sodium carboxymethyl starch, and...
Embodiment 2
[0036] Embodiment 2: the preparation of thalidomide intravenous injection:
[0037] Thalidomide is provided by Changzhou Pharmaceutical Factory of Shanghai Pharmaceutical Group, and the preparation method of Thalidomide intravenous injection is as follows:
[0038] 1) Place co-solvent ethylenediamine in water for injection cooled to room temperature, and mix well;
[0039] 2) Measure thalidomide and co-solvent ethylenediamine according to the mass ratio of thalidomide and co-solvent ethylenediamine as 1:0.23, place thalidomide in co-solvent ethylenediamine solution, stir until completely dissolved;
[0040] 3) Weigh hydroxypropyl-β-cyclodextrin according to the mass ratio of thalidomide to hydroxypropyl-β-cyclodextrin as 1:2.5, and add hydroxypropyl-β-cyclodextrin to step (2 ) in the prepared solution, stirred until completely dissolved;
[0041] 4) Add water for injection to the full amount;
[0042] 5) filling and sealing the medicinal solution in a brown ampoule;
[00...
experiment example
[0045] 1. Method
[0046] From May 2018 to July 2019, a total of 100 participants were enrolled in 6 centers in South China, and randomly received thalidomide (Example 1) (group A, N=49) or Placebo (Group B, N=50) treatment. A total of 99 patients have completed. Patients were followed for a median of 156.56 days (range, 16 to 360 days), and 99 people were included in the final analysis.
[0047] 2. Usage:
[0048] The test drug was started at 100 mg / day, taken after dinner. For patients who can tolerate it, the dose is increased to 150 mg per day after 3 days. If necessary, the dose should be adjusted according to adverse reactions.
[0049] 3. Research objectives
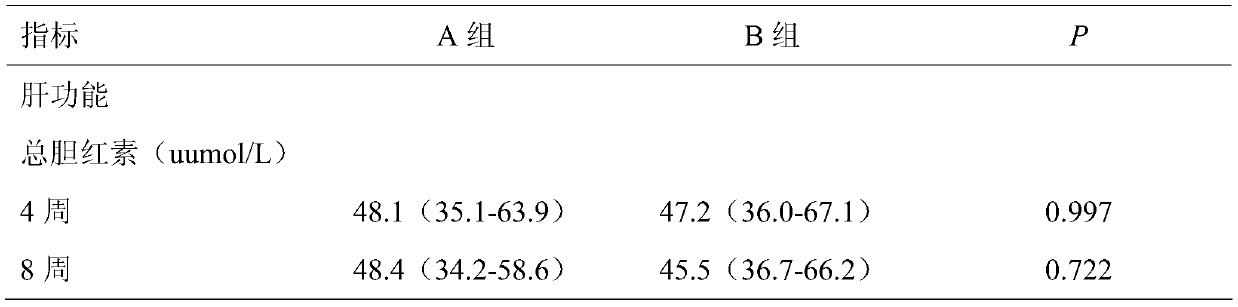
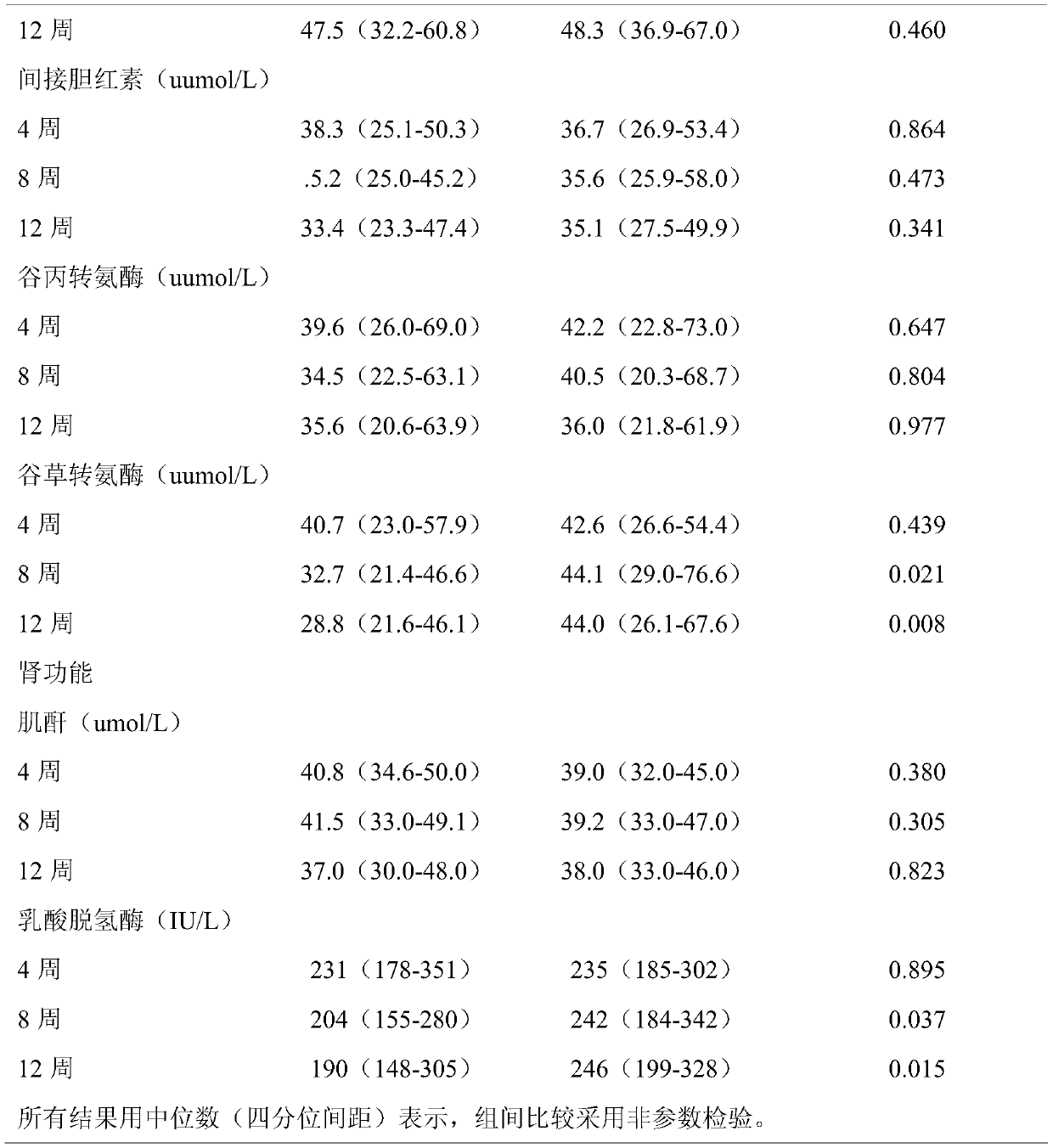
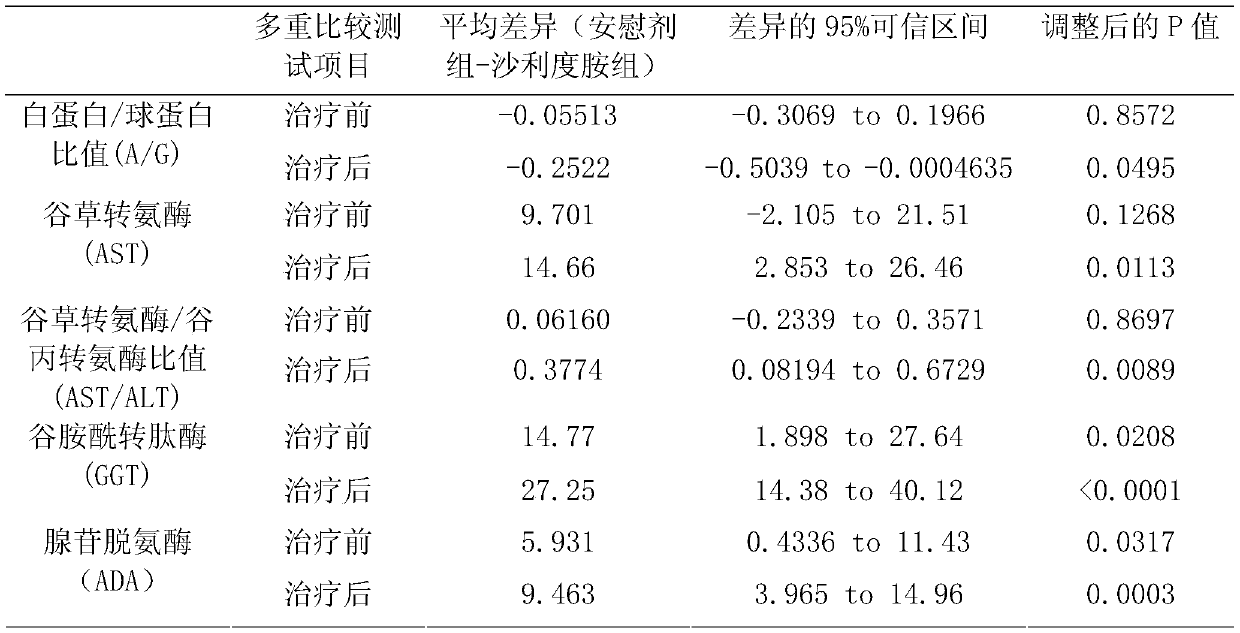
[0050] The main observation goal is the change of hemoglobin level from baseline to week 12. The observation goals include comparing blood transfusion volume, change of HbF level, red blood cell lifespan, liver and kidney function myocardial enzymes, and evaluating safety at the end of follow-up.
[0051] 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com