PCR amplification primer set, amplification reagent and kit for rapidly detecting Thailand alpha-thalassemia
A technology for thalassemia and amplification primers, applied in the field of kits for single-tube direct PCR detection of Thai-type α-thalassemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
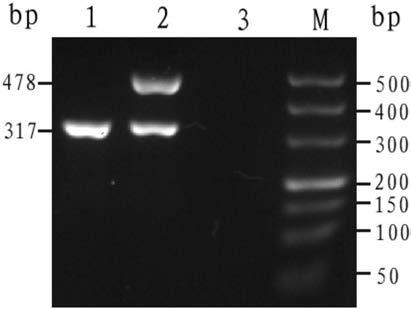
[0082] Embodiment 1: The detection result of using the kit of the present invention in whole blood samples with known genotypes
[0083] 1. The composition of the kit:
[0084] Can amplify the alpha-globin gene cluster -- THAI The primer set of the characteristic sequence of allele and normal gene (NG_000006.1)
[0085] THAIF, THAIR, APF and APR:
[0086] Table 2 Detection-- THAI Primer sets for thalassemia-characteristic sequences
[0087]
[0088] Preferably, the PCR reaction system is prepared according to the following Table 3 (primer working concentration is 10 μmol / L, MightyAmp DNAPolymerase is 1.25U / μL):
[0089] Using the orthogonal test method, through a large number of experimental comparisons, and through a large number of experimental comparisons, the optimal PCR reaction solution formula and system are finally determined, as shown in Table 3:
[0090] 18 μl of PCR reaction solution, 2 μl of samples such as peripheral blood (villi, amniotic fluid or umbilic...
Embodiment 2
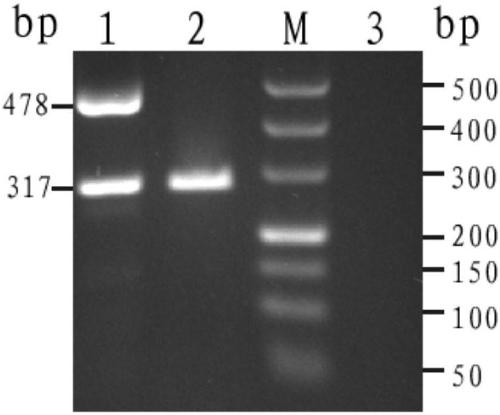
[0100] Example 2: The detection effect of the kit of the present invention in DBS samples.
[0101] 1. The composition of the kit:
[0102] With embodiment 1.
[0103] Preferably, the PCR reaction system is prepared according to Table 3:
[0104] Using the orthogonal test method, through a large number of experimental comparisons, the optimal PCR reaction solution formulation system is finally determined in Table 3: PCR reaction solution 18 μl, DBS sample 1-3 pieces (diameter 1-2mm) (replenish water 2 μl), the total reaction volume was 20 μl.
[0105] 2. Implementation method:
[0106] With embodiment 1.
[0107] 3. Sample source:
[0108] All samples were derived from anticoagulated peripheral blood samples whose genotypes were determined by conventional Gap-PCR technology, and were collected and prepared through filter paper dried blood spot samples. A double-blind experiment was used for detection. Cut a small disc (diameter: 1-2 mm) in the sample area with a punch. ...
Embodiment 3
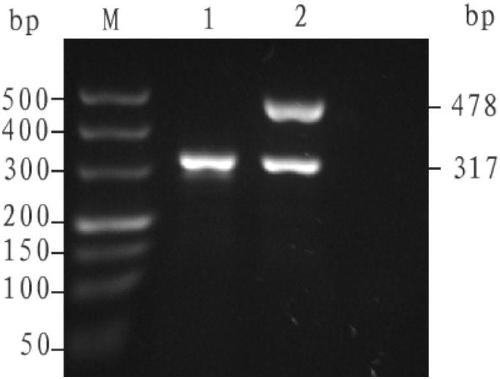
[0111] Example 3: The detection effect of the kit of the present invention on amniotic fluid samples (or cultured amniotic fluid samples).
[0112] 1. The composition of the kit:
[0113] With embodiment 1.
[0114] Prepare the PCR reaction system according to Table 3:
[0115] 2. Implementation method:
[0116] With embodiment 1.
[0117] 3. Sample source:
[0118] All samples were derived from amniotic fluid samples whose genotypes were determined by conventional Gap-PCR technique.
[0119] 4. Data analysis and result judgment: the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com