In vitro cell culture methods for beta-thalassemia using activin type ii receptor ligand traps
An in vitro cell culture, activin receptor technology, applied in cell culture active agents, receptors/cell surface antigens/cell surface determinants, chemical instruments and methods, etc., can solve problems such as unmet medical needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0380] 8.1 Example 1. Erythropoietic responses of ligand wells for activin receptors in culture from β-thalassemia patients
8.1.1 Background technology
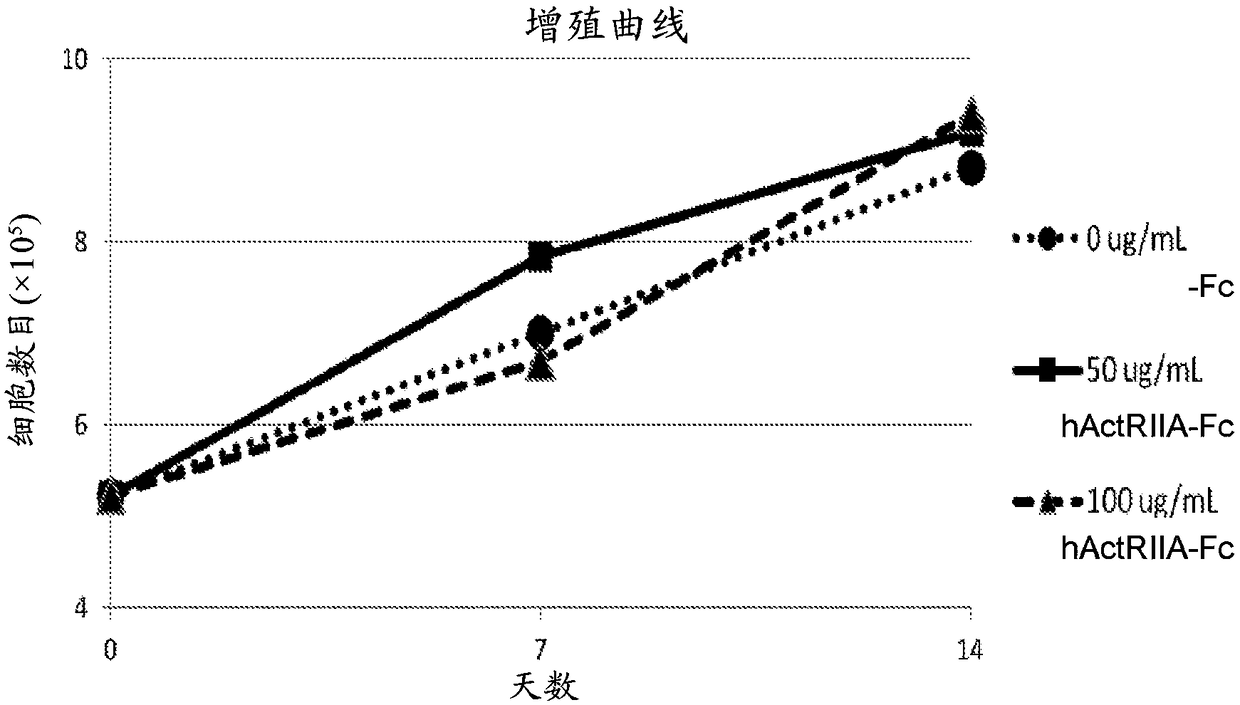
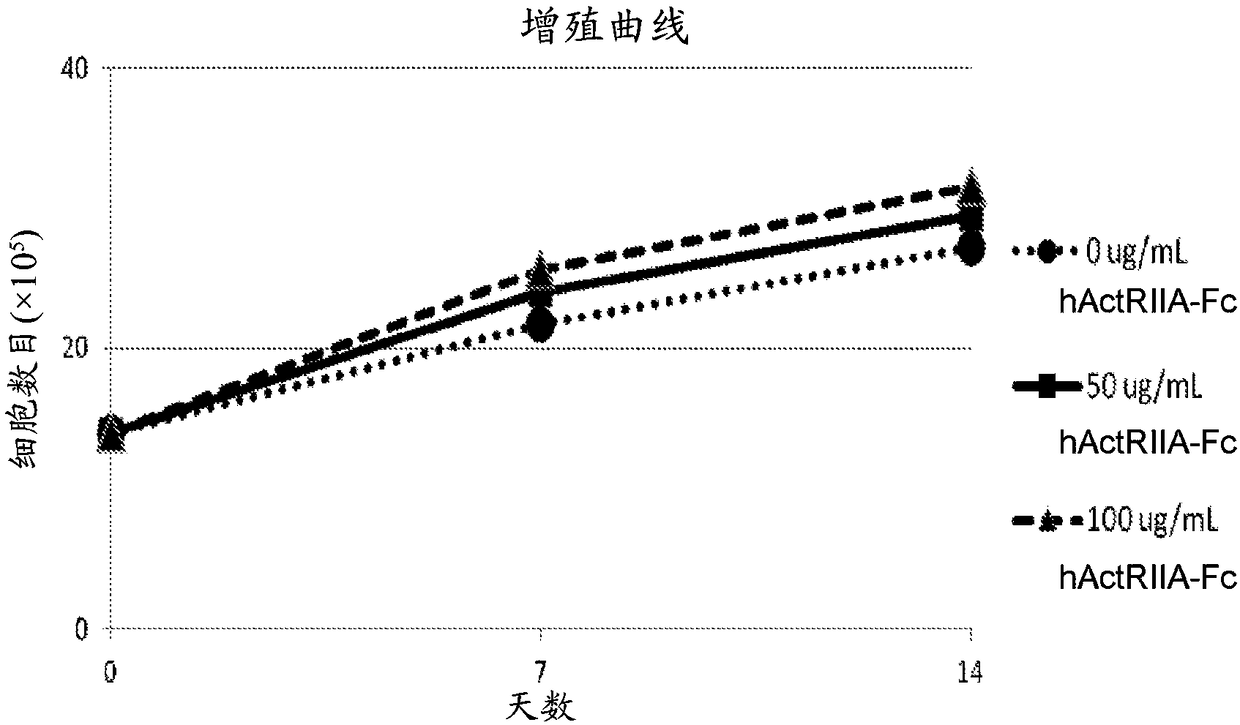
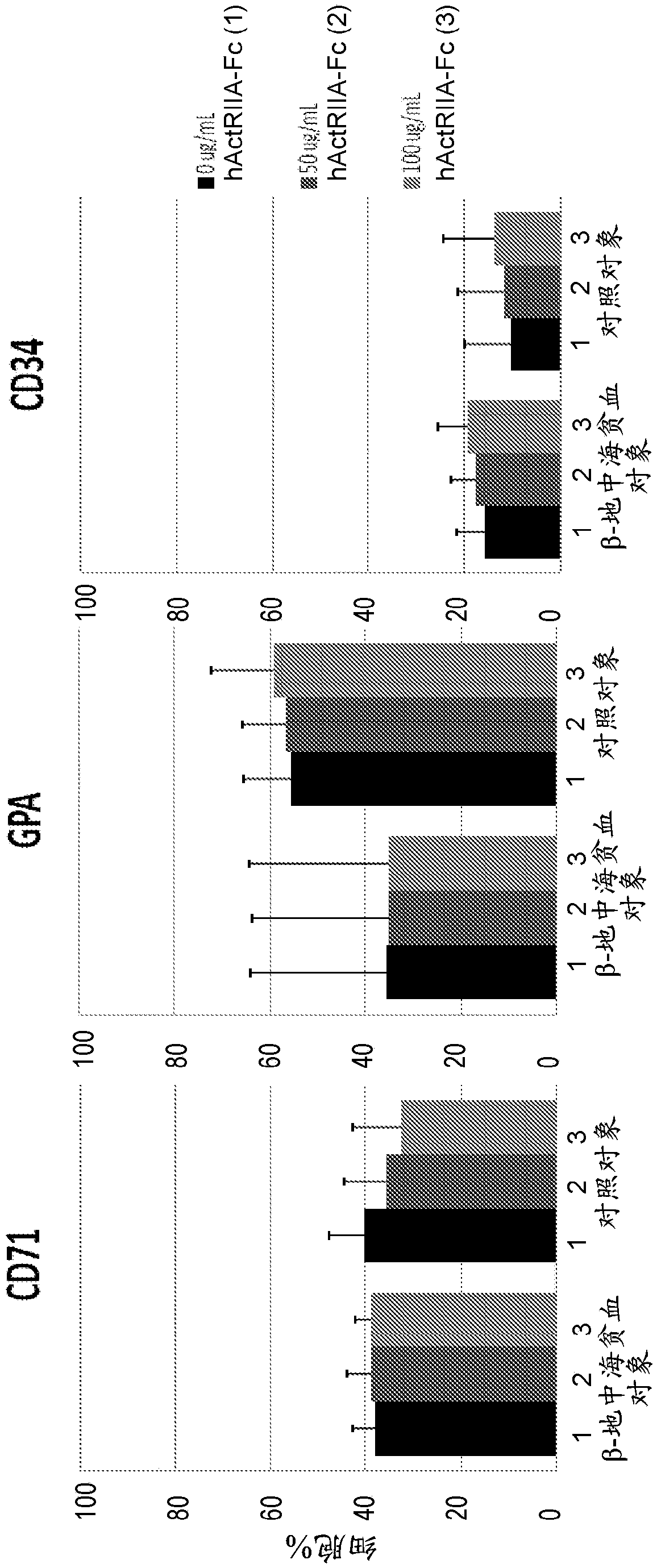
[0381] The hallmark of beta-thalassemia is ineffective erythropoiesis leading to anemia and tissue hypoxia. Activins have been shown to affect erythropoiesis in late maturation. ActRIIA-hFc (SEQ ID NO:7) is a recombinant type IIA activin receptor (ActRIIA) ligand trap that binds activin A / B and other transforming growth factors with high affinity. In animal models, ActRIIA-hFc (SEQ ID NO:7) reverses bone loss and increases hemoglobin and hematocrit through mechanisms that are not fully understood.
[0382] This example studies the effect of ActRIIA-mFc (see, e.g., U.S. Patent No. 8,173,601 and Carrancio et al., 2014, British Journal of Haematology, 165:870-882) on different stages of differentiation and maturation from β-thalassemia patients. Molecular mechanisms underlying the effects on erythropoiesis.
[0383] 8.1.2 M...
Embodiment 2
[0415]8.2 Example 2. Research on the regulation of erythropoiesis by Sotatercept (ACE-011) in human normal and β-thalassemia erythrocyte liquid culture system
[0416] 8.2.1 Introduction
[0417] This example provides a more detailed description of some of the experiments described in Example 1 (Section 8.1) and others compared to Example 1 (Section 8.1).
[0418] 8.2.2 Materials and methods
[0419] (a) CD34 + Cell Separation
[0420] Peripheral blood obtained from 5 β-thalassemia patients and 5 healthy donors and prepared CD34-enriched by using lymphocyte separation medium (Cappel, Aurora, OH) + Cell. CD34 was positively selected by a small MACS immunomagnetic separation system (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions + cell. Briefly, to obtain normal CD34 + cells, will be 10 8 Mononuclear cells of 1 or less were washed twice, and then suspended in 300 L of sorting buffer consisting of 1× phosphate-buffered saline (PBS), 2 mM EDTA (e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com