Composition for treating brain lesions
A composition and polymer technology, applied in the direction of drug combination, medical raw materials derived from mammals, drug delivery, etc., can solve the problems of restricting the introduction of grafts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
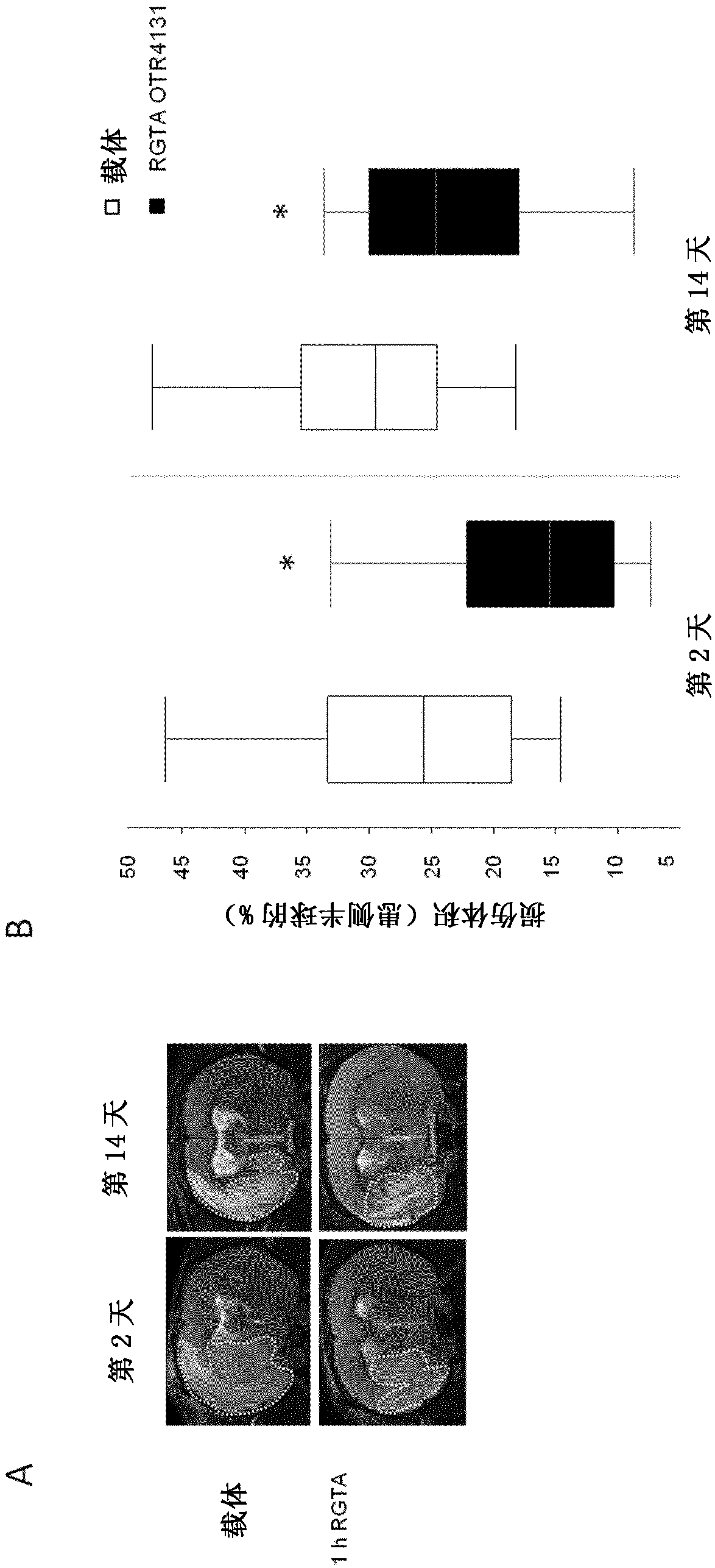
[0133] Example 1: Evaluation of the effect of biocompatible polymers according to the invention on brain damage and functional deficits caused by cerebral ischemia
[0134] In this example, the biocompatible polymer is the polymer sold by the company OTR3 under the trade number OTR 4131, which is described in Frescaline G. et al., Tissue Eng Part A. 2013 Jul; 19(13-14): 1641-53.doi:10.1089 / ten.TEA.2012.0377 and is commercially available.
[0135] Rats were Sprague Dawley strain male rats.
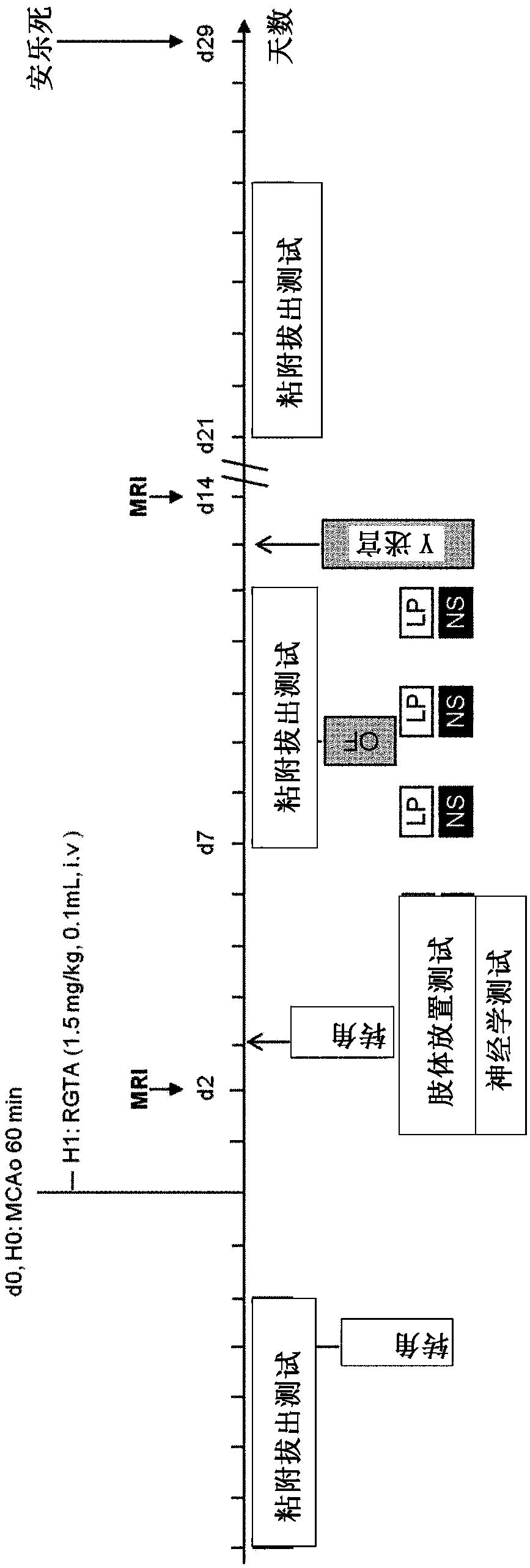
[0136] To define the effect of OTR 4131 biocompatible polymer on brain damage and functional deficits, in rats with transient cerebral ischemia caused by occluded middle cerebral artery figure 1 Experimental protocol shown.
[0137] Specifically, by inhaling O 2 / N 2Animals were anesthetized with 5% isoflurane in O mixture (1 / 3 ratio each) for 3 min and then maintained by mask delivery of 2-2.5% isoflurane during surgery. Lay the rat on its back. The incision is made at the level of t...
Embodiment 2
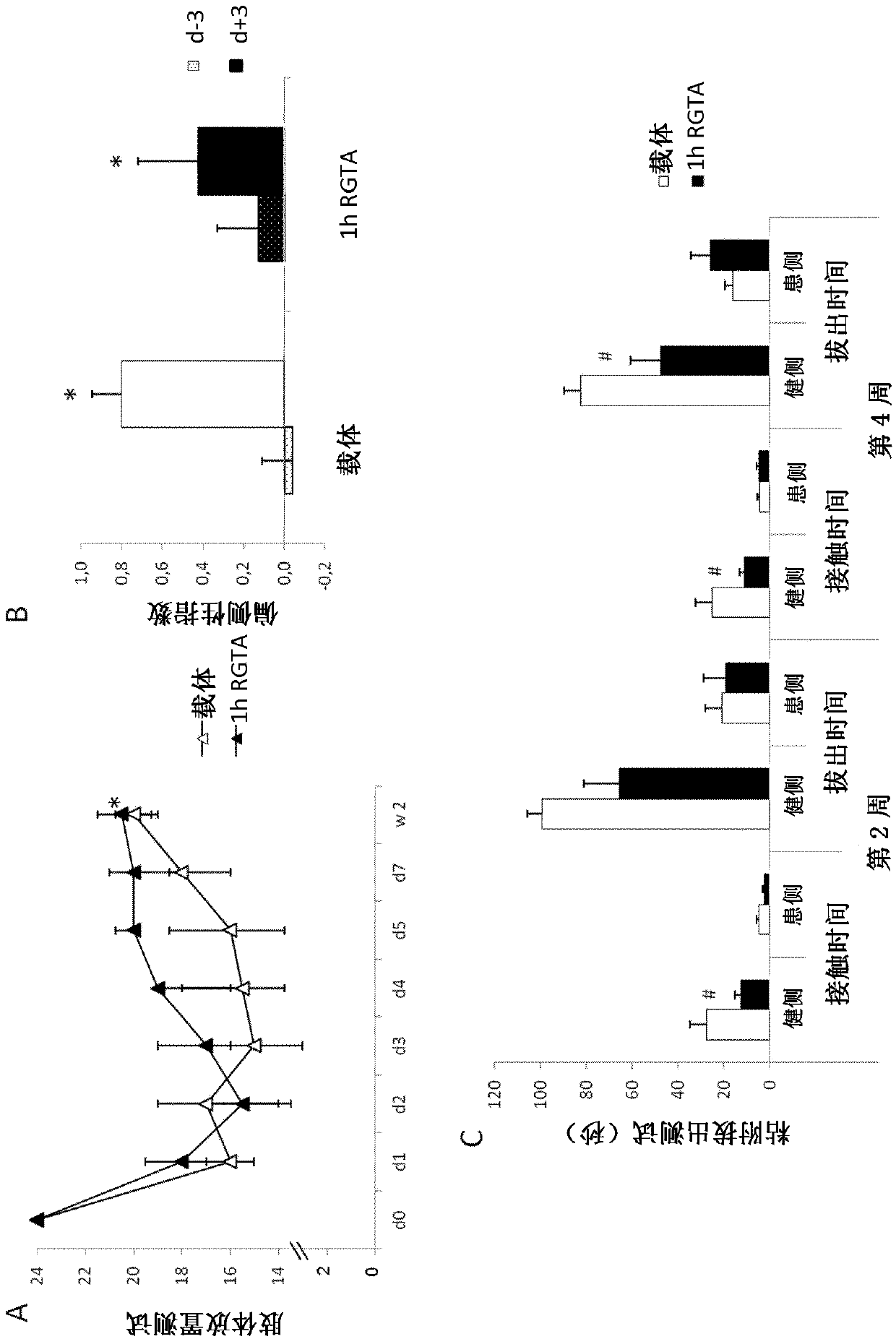
[0145] Example 2: Evaluation of the effects of co-administration of biocompatible polymers and mesenchymal stem cells on brain damage and functional deficits caused by ischemic shock
[0146] In this example, rats and biocompatible polymers were the same as those in Example 1.
[0147] According to the method described in the document Quittet et al., "Effects of mesenchymal stem cell therapy, inassociation with pharmacologically active microcarriers releasing VEGF, in anischaemic stroke model in the rat" Acta Biomater. 2015 Mar; 15:77-88, by Sprague Dawley Mesenchymal stem cells were extracted from rat femur and tibia.
[0148] In order to define the effect of co-administration of OTR 4131 biocompatible polymer and mesenchymal stem cells on brain damage and functional deficits, following the intraluminal method as described in Example 1 above, in transient in ischemic rats Figure 4 The experimental protocol shown in.
[0149] Evaluation of the effect of coketone administra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com