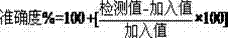Method for determining impurity content of apixaban by virtue of high performance liquid chromatography
A high-performance liquid chromatography and apixaban technology, which is applied in the field of high-performance liquid chromatography for determining the impurity content of apixaban, and achieves the effects of accurate and reliable determination results and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The method specificity test of the present invention:
[0018] Examine the specificity of the method by injecting the test solution containing all potential impurities as the specific solution. Determine the relative retention time and separation degree of each potential impurity under the determination conditions through the specificity test, to ensure that the impurities of concern can be detected, to ensure that the blank solution has no interference at the retention time of apixaban and impurities, and to ensure that the apixaban The peak purity of Banfeng is pure.
[0019] Result: In the blank solution, there is no interference peak at the retention time of the impurity and apixaban peaks; in the specific solution, all peaks should be separated from the known impurities and apixaban peaks, each known impurity and apixaban peak The separation degree of its adjacent peak is ≥1.5; in the test solution under degradation conditions, the separation degree between two re...
Embodiment 2
[0025] The system suitability test of the inventive method:
[0026] On every working day during the method validation, the system suitability test is carried out before the validation starts. Report system suitability results, with retention times for known impurities and apixaban. In the system suitability solution tested every day, the tailing factor of the apixaban peak should not be greater than 1.5; in the control solution, the theoretical plate number of the apixaban peak should not be lower than 40,000, and the signal-to-noise ratio should not be lower than 30.
[0027] .
Embodiment 3
[0029] The accuracy test of the inventive method:
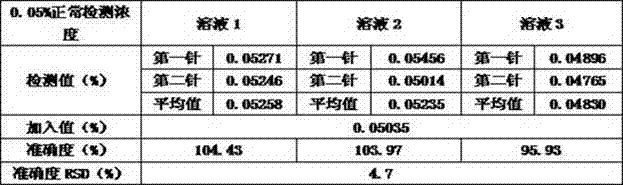
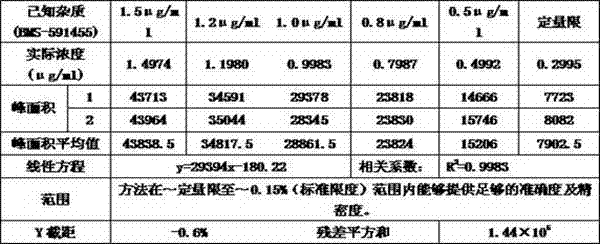
[0030] The accuracy of the method is achieved by examining the accuracy of adding different concentrations of known impurities to the sample. Investigate the concentration of known impurities including quantification limit concentration (6 parts), 0.5μg / ml known impurities (0.05%), 1.0μg / ml known impurities (0.10%), 1.5μg / ml known impurities (0.15%) , each concentration was repeated 3 times, and the concentrations of unknown impurities were investigated, including the limit of quantification, 0.5 μg / ml apixaban (0.05%), 1.0 μg / ml apixaban (0.10%), 1.5 μg / ml apixaban Shaban (0.15%), each concentration was repeated 3 times. When the concentration is 0.10% and 0.15% of the normal detection concentration, the relative standard deviation (RSD) of the accuracy result is ≤15%, and the accuracy result should be 100±15%; when the concentration is 0.05% of the normal detection concentration, the relative standard deviation (RSD) of th...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap