A kind of preparation method of thiocyanate compound
A technology of ester compounds and thiocyanate, applied in organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve problems such as heavy metal residues in thiocyanate compounds, affecting the application of thiocyanate compounds, etc. Achieve the effect of avoiding adverse effects and easy removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The invention provides a preparation method of a thiocyanate compound, including:
[0051] Mixing a mercapto compound, thiocyanate, a catalyst and a polar organic solvent to obtain a raw material mixture, wherein the catalyst is rose bengal, eosin Y or eosin B;
[0052] The raw material mixture is subjected to light reaction under light conditions to obtain thiocyanate compounds.
[0053] The invention mixes mercapto compound, thiocyanate, catalyst and polar organic solvent to obtain a raw material mixture.
[0054] In the present invention, the structural formula of the mercapto compound is R-SH, and the R is preferably an aryl group, a benzyl group or an azacyclic group. The aryl group is preferably alkyl-substituted phenyl, alkoxy-substituted phenyl, 4-amino-substituted phenyl, 4-hydroxy-substituted phenyl, 4-nitro-substituted phenyl, 4-acetyl Substituted phenyl, halogen substituted phenyl, 3,5-bis(trifluoromethyl)-phenyl, benzyl, naphthyl, 4-methoxybenzyl, 4-chlorobenzyl; ...
Embodiment 1
[0075] Add 0.3mmol of ammonium thiocyanate, 0.1mmol of p-methyl thiophenol, rose bengal with a molar content of 1 mol% of ammonium thiocyanate, and 1 mL of acetonitrile into the reaction flask. Under stirring conditions, use 6000k white light As a light source, the raw material mixture is illuminated. After 12 hours of light reaction, the target product is separated and purified by column chromatography after the reaction is completed. The column chromatography eluent used is petroleum ether and ethyl acetate in a volume ratio of 5:1 The mixed solvent, the yield is 85%, and the product purity is 99.8%.
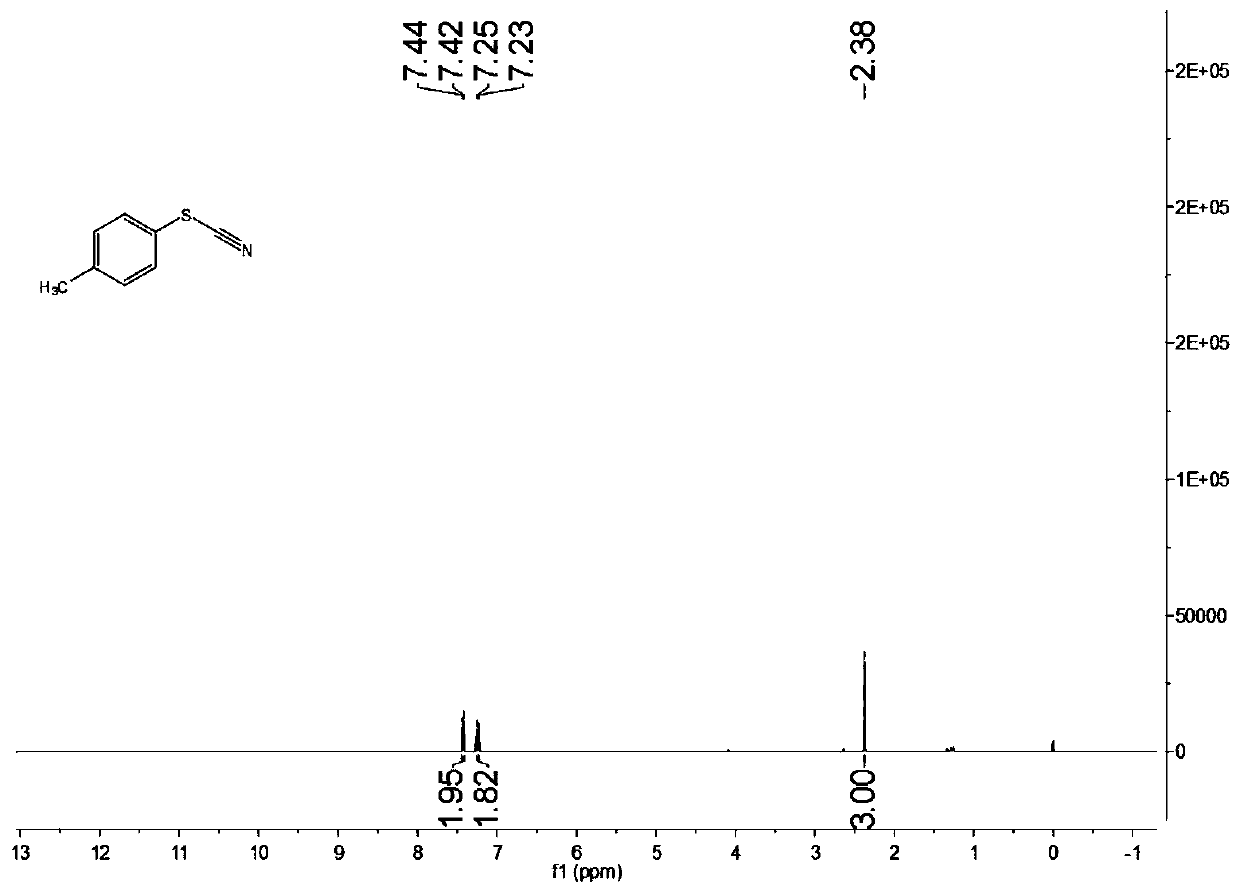
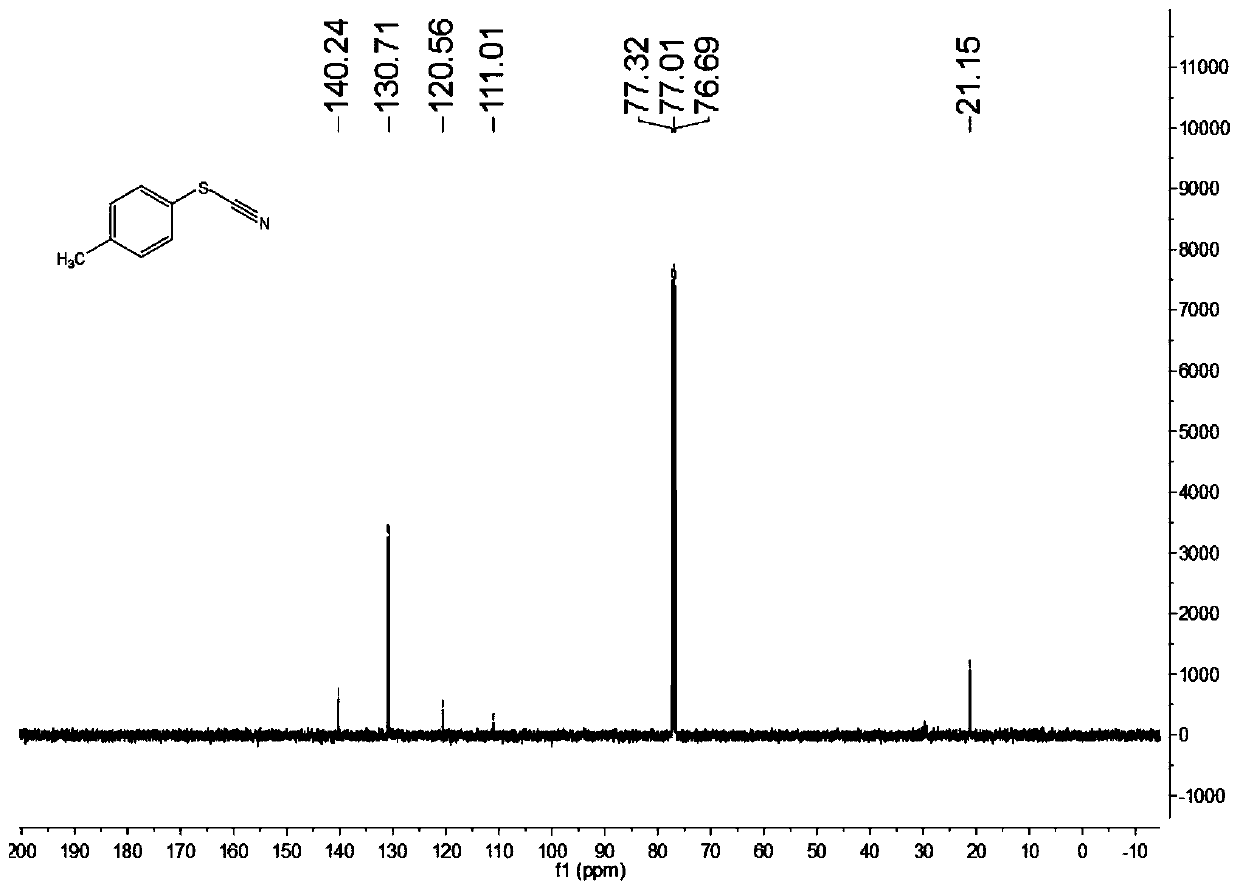
[0076] Characterize the structure of the obtained product, the proton nuclear magnetic resonance spectrum and the carbon nuclear magnetic resonance spectrum are as follows: figure 1 with figure 2 As shown, the structural characterization data is as follows:
[0077] 1 H NMR(400MHz, CDCl 3 ): δ7.43(d,J=8.0Hz,2H), 7.24(d,J=8.0Hz,2H), 2.38(s,3H)ppm.
[0078] 13 C NMR(100MHz, CDCl 3 ):...
Embodiment 2
[0083] Add 0.3mmol of ammonium thiocyanate, 0.1mmol of p-methoxy thiophenol, 1.2 mol% of rose bengal, and 1 mL of acetonitrile into the reaction flask. The white light is the light source, and the raw material mixture is illuminated. After 11 hours of light reaction, after the reaction, the target product is separated and purified by column chromatography. The column chromatography eluent used is 10:1 petroleum ether and acetic acid in volume ratio The mixed solvent of ethyl ester has a yield of 49% and a product purity of 99.7%.
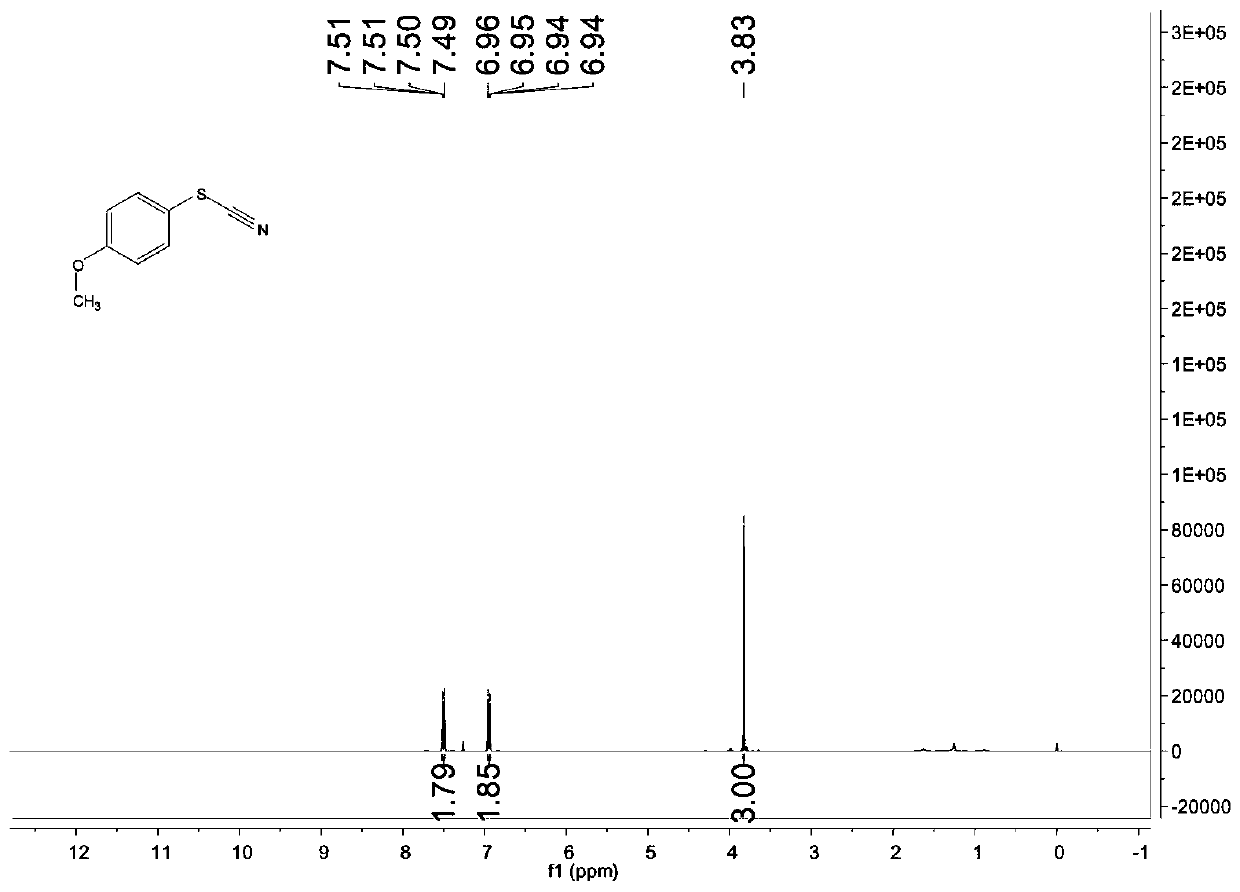
[0084] Characterize the structure of the obtained product, the proton nuclear magnetic resonance spectrum and the carbon nuclear magnetic resonance spectrum are as follows: image 3 with Figure 4 As shown, the structural characterization data is as follows:
[0085] 1 H NMR(400MHz, CDCl 3 ): δ7.51-7.49 (m, 2H), 6.96-6.94 (m, 2H), 3.83 (s, 3H) ppm;
[0086] 13 C NMR(100MHz, CDCl 3 ): δ161.3, 133.8, 115.9, 113.8, 111.6, 55.6ppm.
[0087] MS (EI, 70eV): m / z ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com