A kind of anti-calcification short peptide and its application
A technology of short peptides and drugs, which is applied in the field of biomedicine to achieve the effect of short length and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1, the operation steps of the preparation method of short peptide:
[0025] The main operation steps of solid-phase short peptide are as follows:
[0026] (1) Weigh 0.5 mmol of Fmoc-Thr(tBu)wang resin with a loading of 0.35 mmol / g, place it in a reaction column, add an appropriate amount of DCM to soak for 30 minutes, remove the DCM, and wash with DMF for 3 times.
[0027] (2) For deprotection, add an appropriate amount of deprotection solution (20% piperidine in DMF) to react for 20 min, remove the deprotection solution, and wash with DMF for 6 times.
[0028] (3) Coupling of amino acids, weigh 3eq of Fmoc-Pro-OH and TBTU, add to the reaction column, use DMF as solvent, add 3eq of DIEA, react for 1h, check whether the reaction is complete by ninhydrin, after the reaction , the reaction solution was removed and washed three times with DMF.
[0029] (4) Steps (2) and (3) are repeated until the last amino acid reaction is completed.
[0030] (5) Deprotection...
Embodiment 2
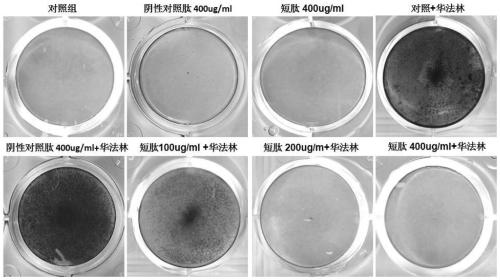
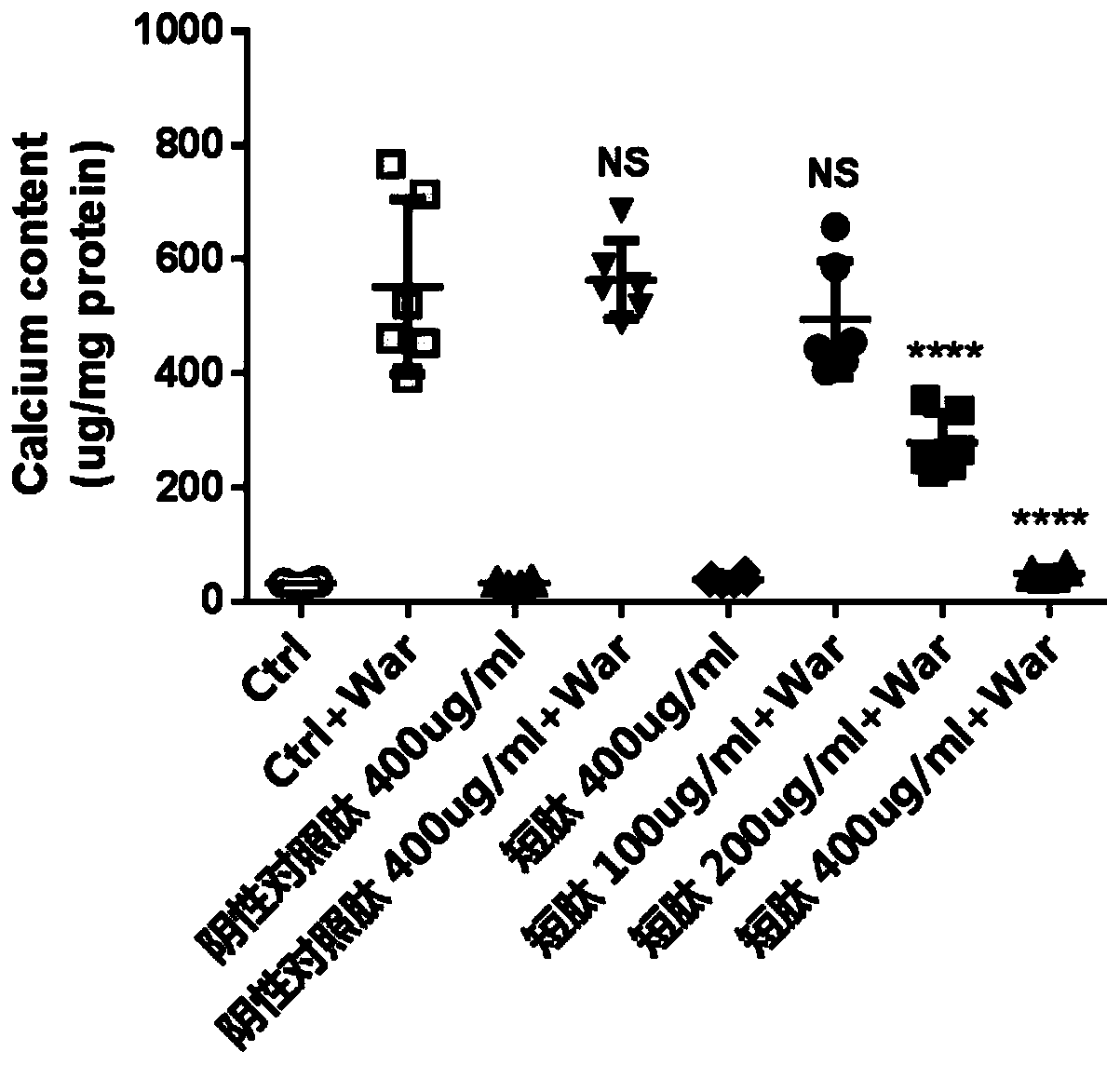
[0033] Example 2, Experiment of Short Peptides Inhibiting Warfarin-Induced Valvular Interstitial Cell Calcification
[0034] Primary porcine aortic valve mesenchymal cells were cultured in DMEM medium containing 10% fetal bovine serum at a cell density of about 1*10 5 The amount of cells per well was added to a 12-well plate, and 1.6mM warfarin solution was added to culture for 7 days. At the same time, different concentrations of the short peptide of the present invention and the negative control peptide (SEQ ID NO.3) were added respectively. The cells were fixed with 4% paraformaldehyde and stained with 1% alizarin red to show the calcium deposition. In addition, 0.6M dilute hydrochloric acid was used to dissolve intracellular calcium, and the colorimetric calcium concentration determination kit was used to detect intracellular calcium. levels of calcium content.
[0035] The results show that the short peptide of the present invention can significantly inhibit warfarin-ind...
Embodiment 3
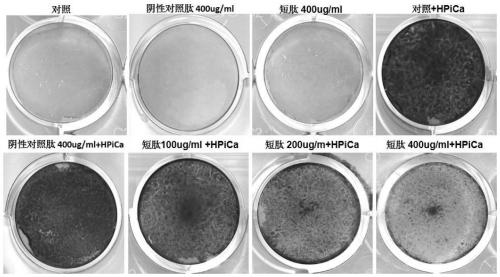
[0036] Example 3, Experiment of Short Peptides Inhibiting Calcification of Valve Interstitial Cells Induced by High Calcium and High Phosphorus
[0037] Primary porcine aortic valve mesenchymal cells were cultured in DMEM medium containing 10% fetal bovine serum at a cell density of about 1*10 5 The amount of cells per well is added to a 12-well plate, 2.0mM calcium chloride and 2.0mM (sodium hydrogen phosphate + disodium hydrogen phosphate) solution are added for 5 days, and different concentrations of short peptides and negative peptides of the present invention are added respectively. For the control peptide (SEQ ID NO.3), the cells were fixed with 4% paraformaldehyde on day 5 and stained with 1% alizarin red to show the calcium deposition, and the intracellular calcium was dissolved with 0.6M dilute hydrochloric acid , using a colorimetric calcium concentration assay kit to detect the level of intracellular calcium content. The results show that the short peptide of the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com