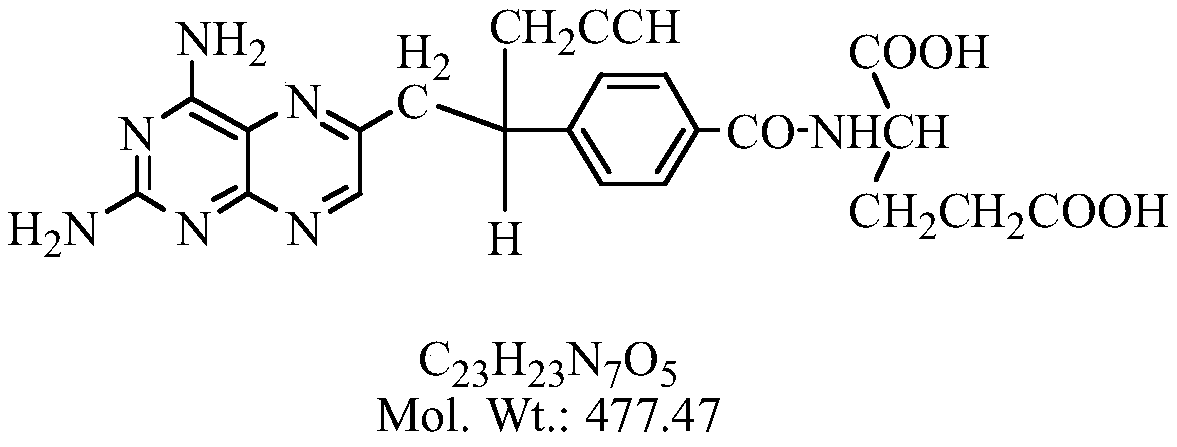
A kind of preparation method of pralatrexate
A technology of temperature control and crystallization, which is applied in the field of medicine, can solve the problems of failing to meet the refining requirements, the increase of impurities, and no refining effect, etc., and achieve the effects of reducing the impact of product quality, high purity and yield, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 20g of PLQS-6 and 100ml of acetone into a 2L three-necked flask and stir. When the temperature is controlled at about 10°C, the bottle becomes a suspension. Start adding 15ml of 1.0mol / L sodium hydroxide aqueous solution dropwise, and control the rate of addition so that the temperature of the reaction flask is 10~13°C, after the dropwise addition is completed, keep stirring for 2 hours, continue to add 15ml of 1.0mol / L sodium hydroxide aqueous solution dropwise, repeat the above operation until all the 91ml of sodium hydroxide aqueous solution is added dropwise, continue to keep stirring for 2 hours, and the reaction solution is clear. Sampling for HPLC detection; the reaction is complete, put the three-neck bottle in a cold bath to cool down to 0-5°C, add 720ml of absolute ethanol dropwise, at this time, a yellow solid precipitates, after the dropwise addition, continue to keep stirring for 1 hour, filter, and add to the filter cake Dissolve in 200ml of purified wa...
Embodiment 2
[0031] Add 20g of PLQS-6 and 200ml of dioxane into a 2L three-neck flask and stir. When the temperature is controlled at about 10°C, the bottle becomes a suspension. Start to add 15ml of 1.0mol / L sodium carbonate aqueous solution dropwise, and control the dropping speed to make the reaction bottle The temperature is 10-13°C. After the dropwise addition is completed, keep stirring for 2 hours, continue to add 15ml of 1.0mol / L sodium carbonate aqueous solution dropwise, repeat the above operation until all the 91ml of sodium carbonate aqueous solution is added dropwise, continue to keep warm and stir for 4 hours, and the reaction solution is clear. Sampling for HPLC detection; the reaction is complete, put the three-neck bottle in a cold bath to cool down to 0-5°C, add 720ml of absolute ethanol dropwise, at this time, a yellow solid precipitates, after the dropwise addition, continue to keep stirring for 1 hour, filter, and add to the filter cake Dissolve in 200ml of purified wat...
Embodiment 3
[0033] Add 20g of PLQS-6 and 60ml of acetone into a 2L three-neck flask and stir. When the temperature is controlled at about 10°C, the bottle becomes a suspension. Start to add 15ml of 1.0mol / L sodium hydroxide aqueous solution dropwise, and control the rate of addition so that the temperature of the reaction flask is 10~13°C, after the dropwise addition is completed, keep stirring for 2 hours, continue to add 15ml of 1.0mol / L sodium hydroxide aqueous solution dropwise, repeat the above operation until all the 91ml of sodium hydroxide aqueous solution is added dropwise, continue to keep stirring for 2 hours, and the reaction solution is clear. Sampling for HPLC detection; the reaction is complete, put the three-neck bottle in a cold bath to cool down to 0-5°C, add 720ml of acetone dropwise, at this time, a yellow solid precipitates, after the dropwise addition, continue to keep stirring for 1 hour, filter, add 200ml of filter cake to purify Dissolve in water, stir with 200ml o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com