A method for the determination of free exenatide by competitive chemiluminescence
A technology of exenatide and chemiluminescence, which is applied in chemiluminescence/bioluminescence, analysis through chemical reaction of materials, biological testing, etc., to achieve the effect of improving sensitivity, increasing sensitivity, and increasing dilution factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Main materials and equipment:
[0053] Molecular Device full-wavelength microplate reader;
[0054] Black bottom opaque chemiluminescence ELISA plate (Nunc, Cat# 437111).
[0055] Biological and chemical reagents:
[0056] Carbonate buffer at pH 9.6
[0057] Phosphate buffer at pH 7.4
[0058] Mouse mAb to Exendin (Abcam, Cat#: ab23407)
[0059] Streptavidin-HRP (21130, Thermo)
[0060] Biotin-conjugated exenatide (Biotin-Exendin 4, 0.1mg / mL)
[0061] Free Exenatide (Exendin-4)
[0062] SuperSignal TM ELISA Femto Substrate (Thermo, Cat #: 37074).
[0063] Method steps:
[0064] 1) Use carbonate buffer (pH9.6) to dilute the mouse anti-exenatide (Exendin-4 1mg / mL) solution to 1 μg / mL, add 100 μL to each well of a 96-well microwell plate, 2- Incubate overnight at 8°C;
[0065] 2) Shake off the liquid in the plate, wash 3 times with PBST (PBS+0.05% Tween-20), 300uL / well, shake for 30sec. Pat dry, add PBS buffer containing 5% skimmed milk, 200uL / well, 37°C, incu...
Embodiment 2
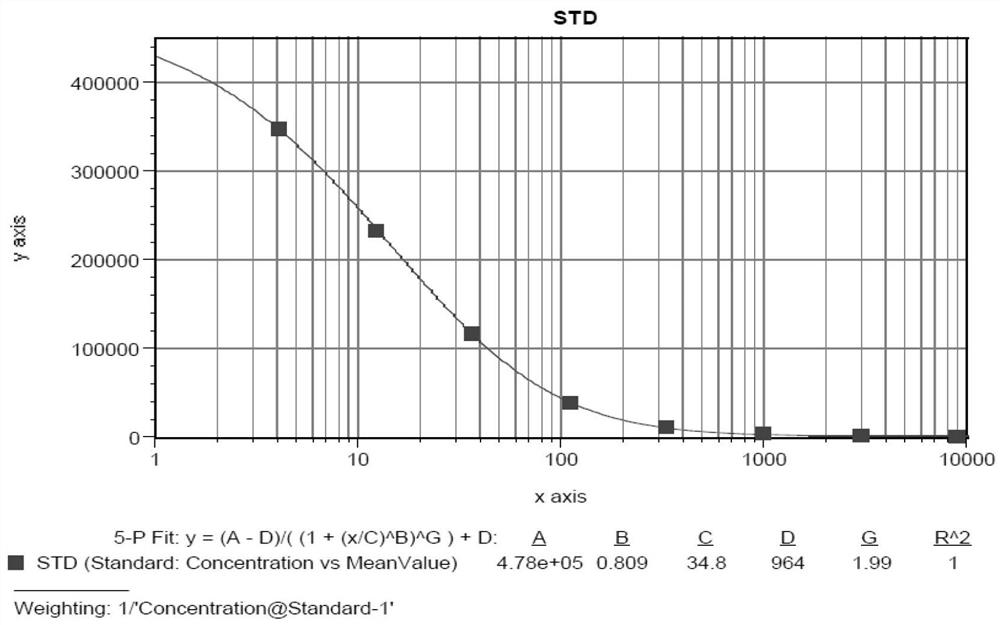
[0075] Standard curve: because the method of the present invention has used competitive reaction mode, so concentration and signal value are in inverse relationship, the proterties of standard curve also present anti-S type, and typical curve sees figure 1 . The standard curves of different analysis batches were verified and compared (see Table 1). The standard curves can be fitted robustly every time, and the differences between different batches are within the range of precision and accuracy requirements, and will not exceed 20% deviation;
[0076]Table 1 Standard curves of different analytical batches
[0077] .
Embodiment 3
[0079] Recovery rate comparison: Add free exenatide to PBS and cell culture medium respectively, the concentration is 8000, 4000, 300, 100, 10, 5pg / mL, measure the recovery rate with reference to the method of Example 1, the recovery rate is In the range of 80% to 120% (see Table 2);
[0080] Table 2 The recovery rate of exenatide in PBS, cell culture medium
[0081] .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com