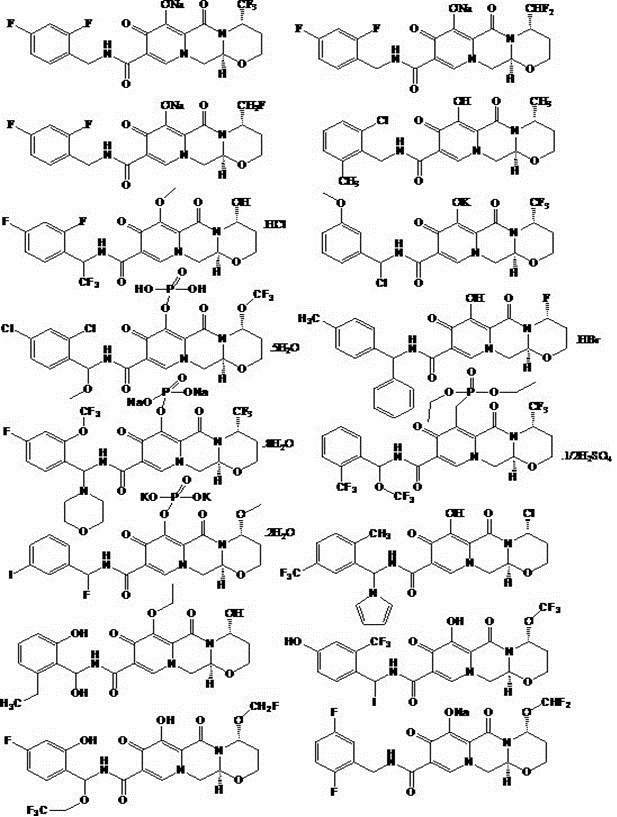
A novel anti-HIV drug and its preparation method and application
A pharmacy and compound technology, applied in the field of new anti-HIV drugs and their preparation and use, can solve problems such as cross-drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
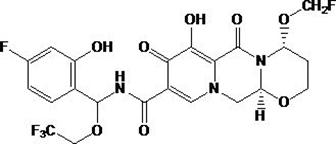
Embodiment 1
[0032] Embodiment 1: the preparation of compound 1
[0033] The reaction equation is as follows:
[0034]
[0035] Under a carbon monoxide atmosphere, a mixture of 33.5 g of compound 1-1, 34.8 ml of diisopropylethylamine, 14.3 mL of compound 2-1 and 4.62 g of tetrakis (triphenylphosphine) palladium dimethyl sulfoxide Stir at 90°C for 5.5 hours. After cooling, the precipitate was filtered and washed with 50 ml of water. Then wash with 500ml of isopropanol, dissolve the solid with ethyl acetate, and wash the organic layer with 335ml of water. The aqueous layer was separated, and 300ml of 0.5N hydrochloric acid was added to the aqueous layer. The aqueous layer was extracted with 300 ml of ethyl acetate. The organic layers were combined and concentrated. To the residue was added 150 ml of isopropanol, cooled to 20°C and filtered, the resulting solid was dissolved in ethanol (100 mL) and treated with 1N sodium hydroxide (aq). The resulting suspension was stirred at room te...
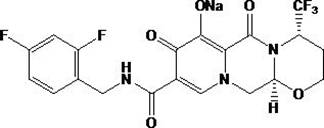
Embodiment 2
[0036] Embodiment 2: the preparation of compound 2
[0037] The reaction equation is as follows:
[0038]
[0039] The preparation method is the same as in Example 1.
Embodiment 3
[0040] Embodiment 3: the preparation of compound 3
[0041] The reaction equation is as follows:
[0042]
[0043] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com