Sulfasalazine crystal form and preparation method thereof
A technology for sulfasalazine crystal and crystal form, which is applied to the crystal form of sulfasalazine and the field of preparation thereof, can solve the problems of not giving up research and the like, and achieve the effects of large crystal particle size and high dry rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
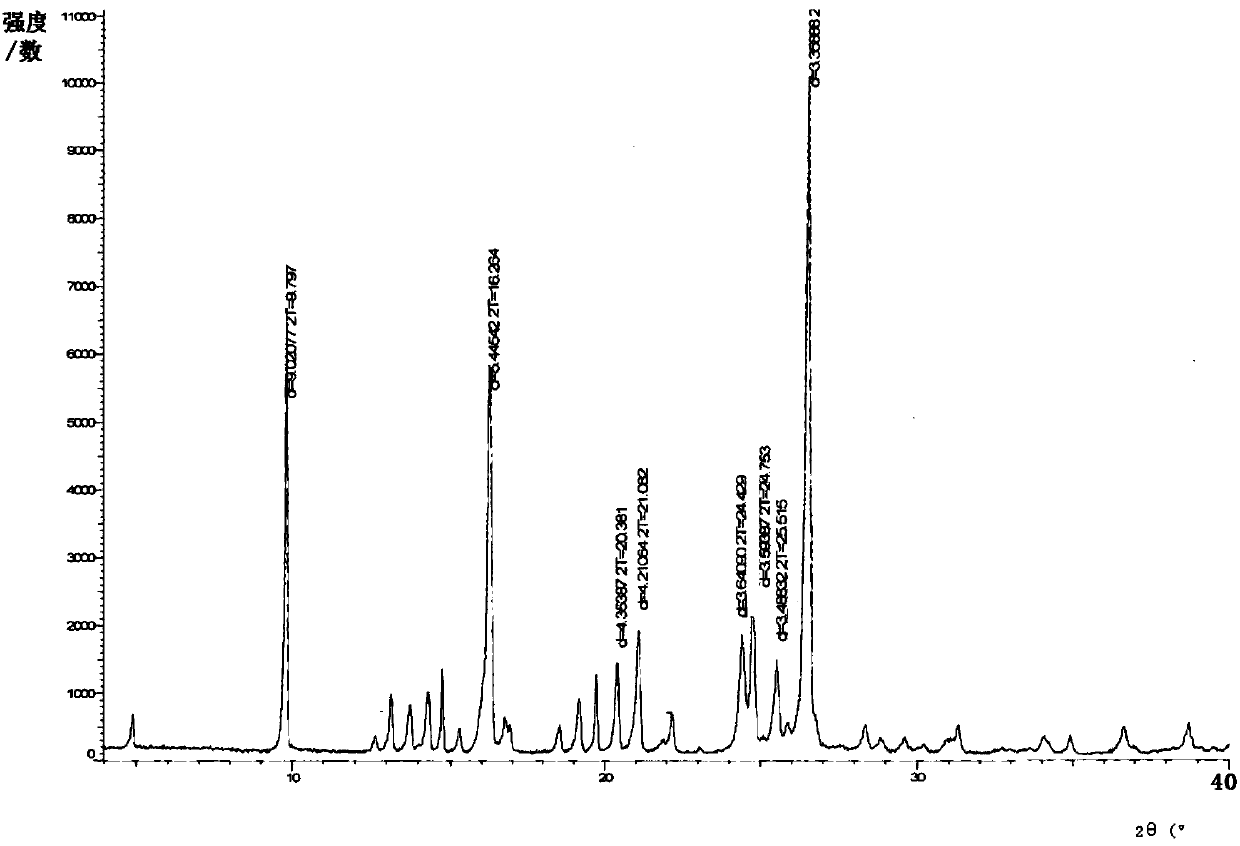
[0040] Add 31g of crude sulfasalazine (wet product) and 50ml of DMF to the reaction flask, stir and heat up to 95-100°C, dissolve it, add 90ml of glacial acetic acid dropwise, keep warm after dropping, and start to cool down to 25- 30°C; after cooling down, heat preservation; after heat preservation, suction filtration and rinsing with 15ml of glacial acetic acid to obtain a wet product; vacuum drying to obtain 11.2g of product, yield 74.7%, HPLC purity 98.1%, after refining, HPLC purity 99.9 %. Powder X-ray Diffraction Pattern See figure 1 , the particle size detection result is D 10 : 6.4 μm, D 50 : 23.8 μm, D 90 : 46.9 μm. Its infrared spectrum is shown in image 3 .
Embodiment 2
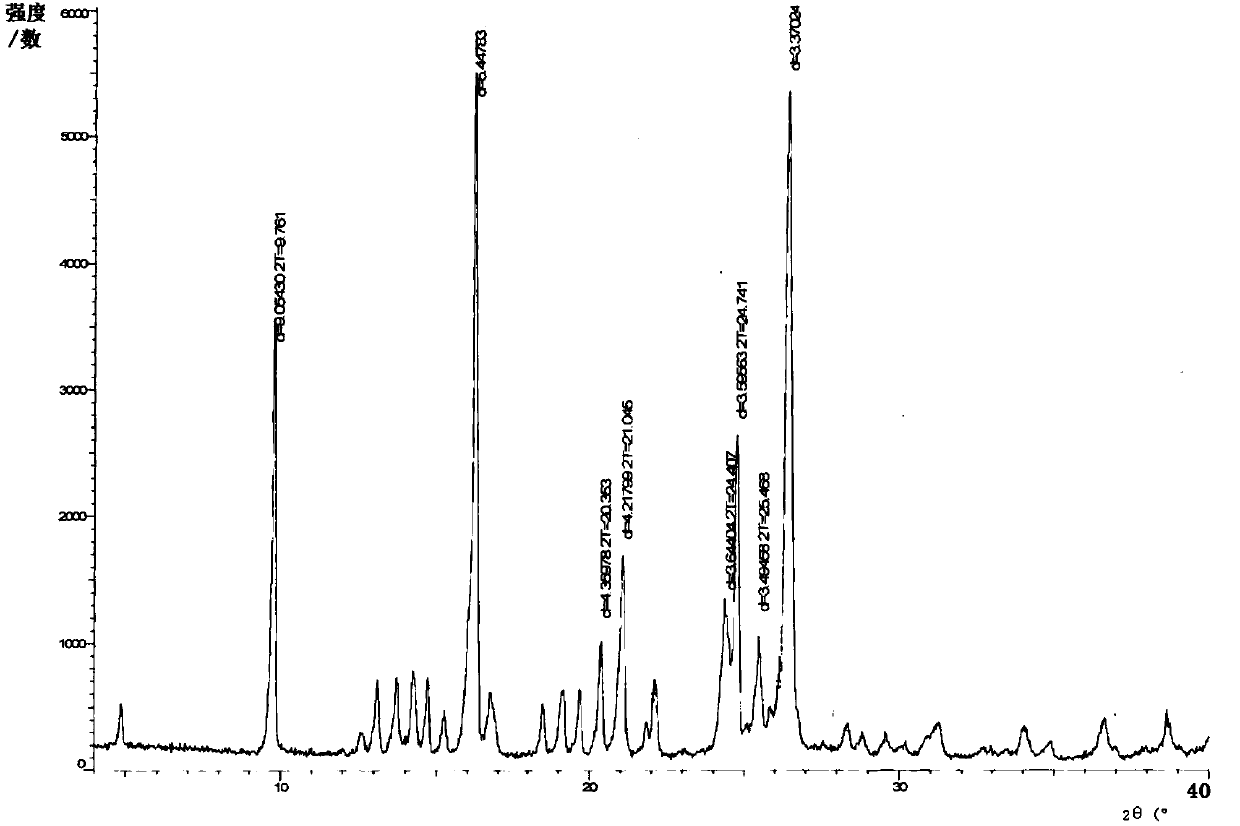
[0042] Add 31g of crude sulfasalazine (wet product) and 50ml of DMF to the reaction flask, stir and heat up to 95-100°C, dissolve it, add 90ml of glacial acetic acid dropwise, keep warm after dropping, and start to cool down to 25- 30°C; after cooling down, heat preservation; after heat preservation, suction filtration and rinsing with 15ml of glacial acetic acid to obtain a wet product; vacuum drying to obtain 11.3g of product, yield 75.3%, HPLC purity 98.0%, after refining, HPLC purity 99.9 %. Powder X-ray Diffraction Pattern See figure 2 , the particle size detection result is D 10 : 5.8 μm, D 50 : 28.7 μm, D 90 : 61.0 μm. Its infrared spectrum is shown in Figure 4 .
Embodiment 3
[0044] Add 31g of crude sulfasalazine (wet product) and 50ml of DMSO to the reaction flask, stir and heat up to 95-100°C, dissolve, add 90ml of propionic acid dropwise, after the dropwise addition, add the new crystal form prepared in Example 1 Seed crystals, heat preservation; after heat preservation, start to cool down to 25-30°C; after cooling down, keep heat; after heat preservation, filter with suction, and rinse with 15ml propionic acid to obtain a wet product; vacuum dry to obtain 10.3g of the product, with a yield of 68% , HPLC purity 98.12%. It was determined by powder X-ray diffraction pattern detection that it was the same as Example 1, both of which were new crystal forms of sulfasalazine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com