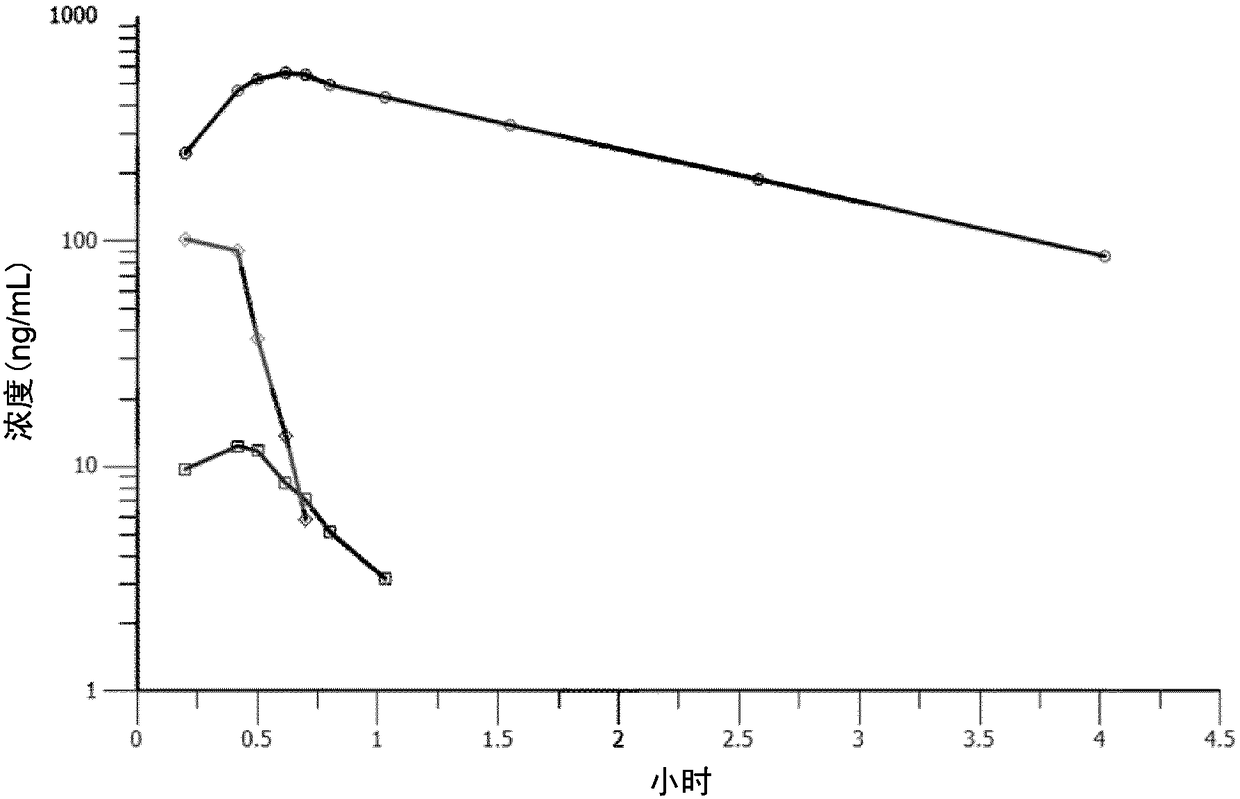
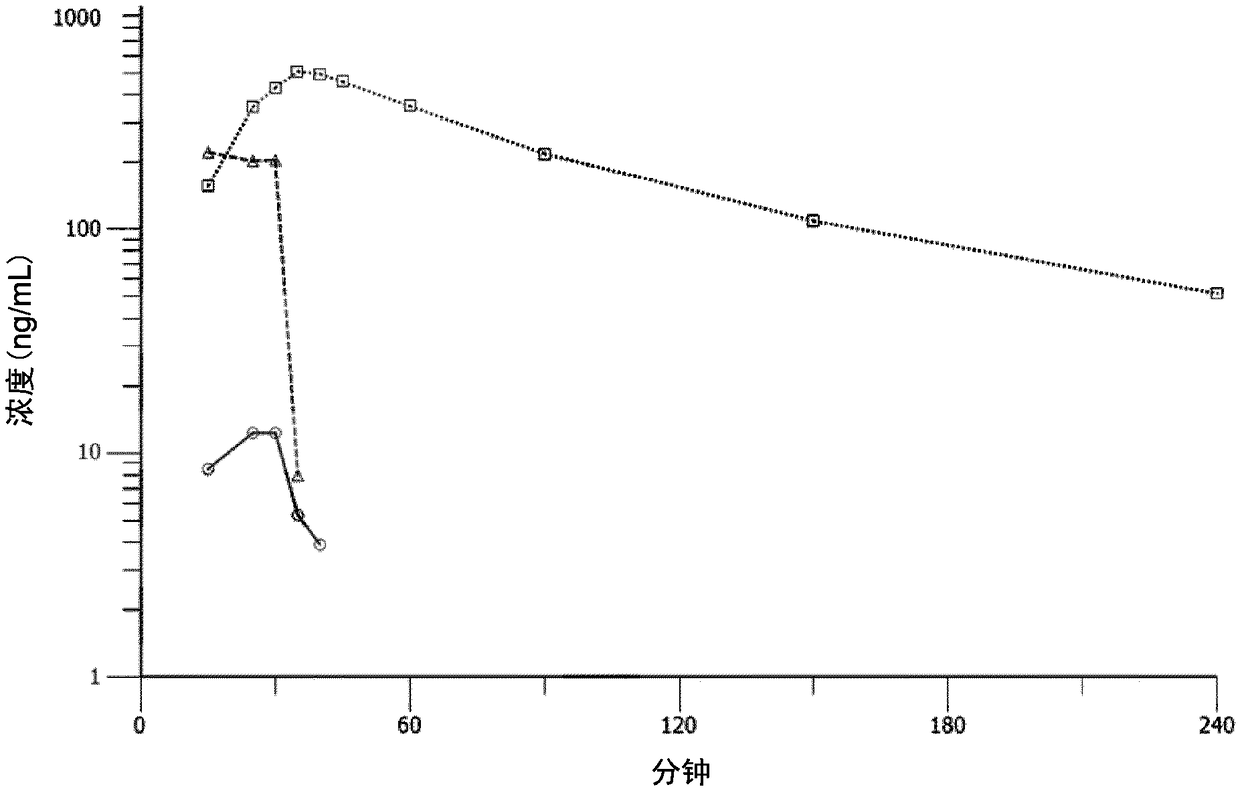
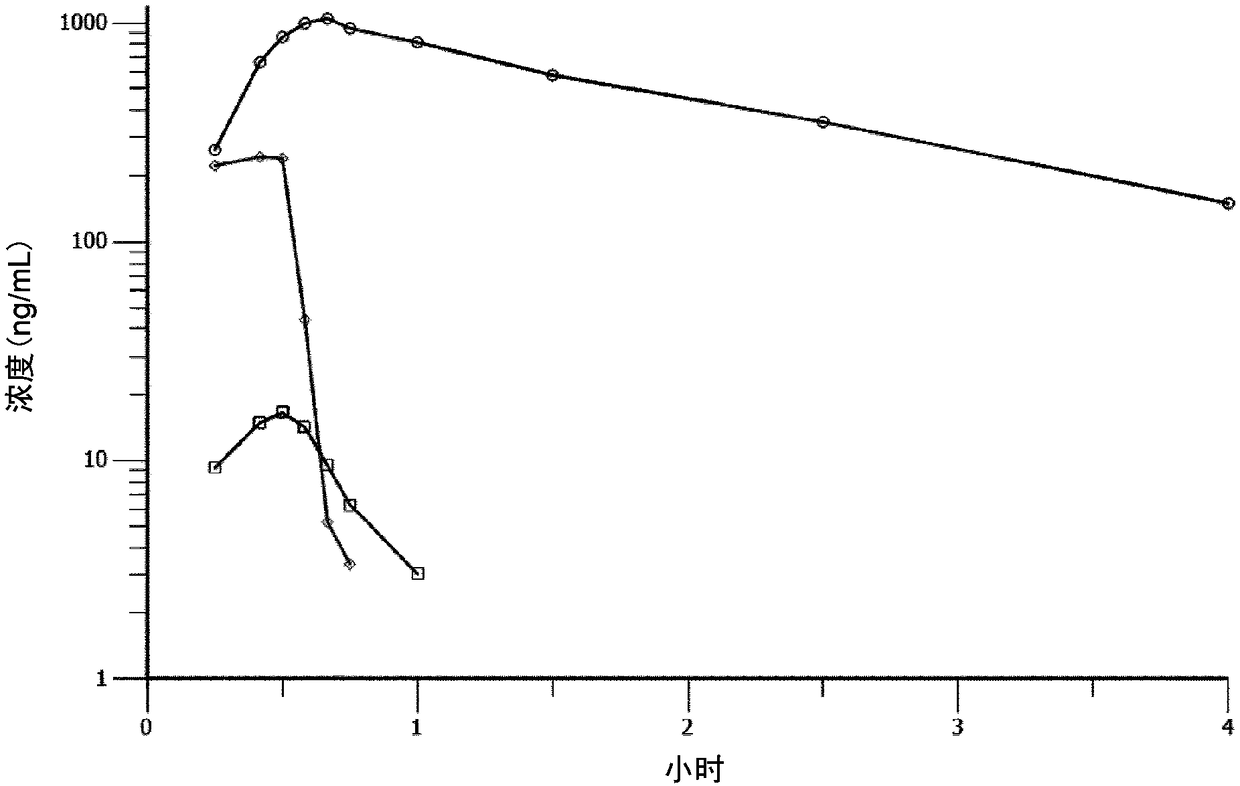
Melflufen dosage regimens for cancer
一种剂量、质量的技术,应用在医药配方、非有效成分的医用配制品、含有效成分的医用配制品等方向,能够解决致命、MM无法治愈等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0039] Example 2 and the aforementioned clinical study reporting PFS included most patients with refractory MM; the results of Example 2 compared favorably with the other studies mentioned above. At the data cutoff point for Example 2b, 29 of the 30 patients in Example 2a had refractory status. Of these 29 patients, 24 (83%) were IMiD refractory, 19 (66%) were PI refractory, and 15 (52%) were alkylating agent refractory. Seventeen (59%) of 29 patients were dual refractory (IMiD and PI), and 9 (31%) were dual and alkylating agent refractory. Twenty-three of 29 patients (79%) were refractory to the most recent line of treatment. The median number of prior treatments for all 40 patients was 4 (range 1 to 13). Available data show a rapid and durable treatment response to the combination of Metraphan with 40 mg weekly dexamethasone. Of the 30 patients evaluated, 19 (63%) reported a best response or better with MR and 12 (40%) reported PR or better.
[0040] As described in Exam...
Embodiment 1
[0206] Example 1: Evaluation of four dose levels (15, 25, 40 and 55 mg) of intravenous metropene hydrochloride (excluding The quality of the salt component) (in combination with dexamethasone treatment on days 1, 8 and 15) for a minimum of 1 cycle and a maximum of 12 cycles.
[0207] Example 2: Open-label single-arm extension at a dose of 40 mg within 30 minutes of Example 1. Administer intravenous metropene (with 40 mg dexamethasone (oral or intravenous) on Days 1, 8, and 15) over 30 minutes on Day 1 of a 21-day cycle or a 28-day cycle for At least 2 cycles, up to the specified number of cycles. During the clinical trial of Example 2, the cycle was extended from 21 days to 28 days according to the protocol modification, in order to better recover neutrophils and thrombus cells before the start of the new cycle.
[0208] The dose reduction from 40 mg to 25 mg metropene in patients in the trial may be related to the adverse effects of thrombocytopenia / neutropenia.
[0209] E...
Embodiment 2a
[0238] Example 2a is the data / results of Clinical Trial Example 2 at time point (a) during the clinical trial.
[0239] 3.1 Efficacy data from studies in patients with RRMM
[0240] Before the data cut-off point of Example 2a, 38 patients with recurrent MM had been given 40 mg of metropene hydrochloride (40 mg dose excluding the mass of the salt component) every 3 weeks (21 days) within 30 minutes with weekly administration of dexamethasone (days 1, 8 and 15). A total of 162 doses of metropene were administered. The median number of cycles started was 3 (1-13), and the median treatment duration was 13 weeks (2-51). The mean dose intensity was 96% (77-100). According to the data cutoff point, 10 patients remained on treatment, 2 completed treatment, and 26 patients discontinued treatment (15 due to AEs, 8 due to PD, 2 deaths, 1 due to other reasons). Twenty-seven patients remained on study (10 patients on treatment, 17 on follow-up), while 11 patients were not on study (8 p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com