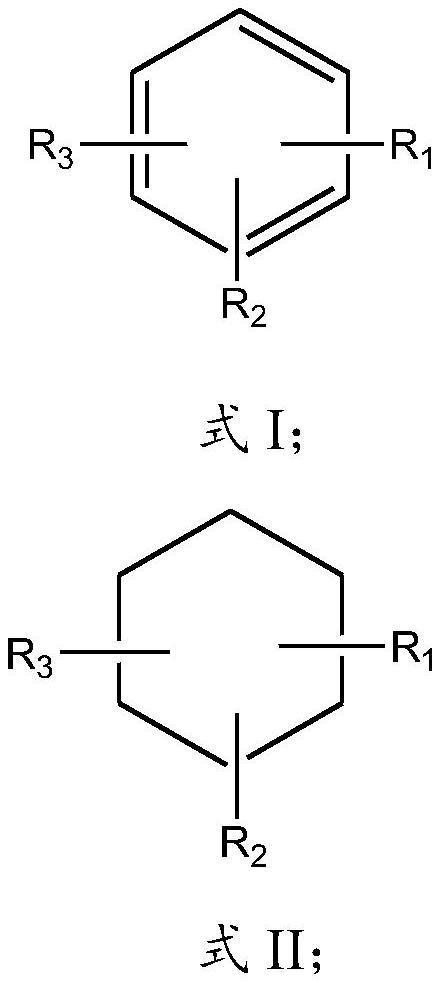
A method for separating iron oxyhydroxide and sulfur and application of iron oxyhydroxide for sulfur removal
A technology for ferric oxyhydroxide and separation of hydroxyl groups, applied in the directions of ferric oxide/ferric hydroxide, separation methods, chemical instruments and methods, etc., can solve problems such as the decrease of desulfurization capacity of ferric oxyhydroxide, and achieve the effect of ensuring desulfurization properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] This embodiment provides a method for separating iron oxyhydroxide and sulfur, which includes the following steps:
[0037] (1) Dry the sulfur-containing iron oxyhydroxide at 10°C to a moisture content of 5wt%;
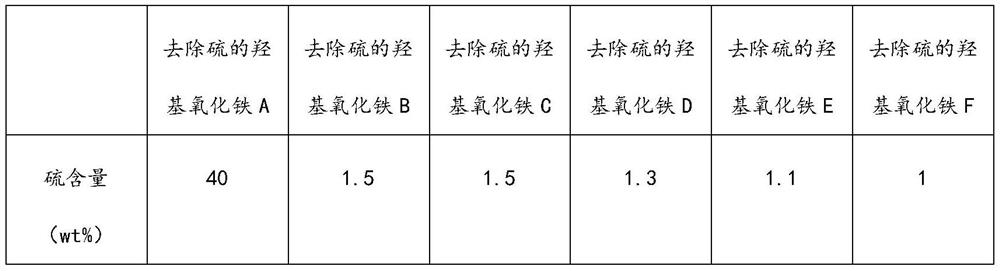
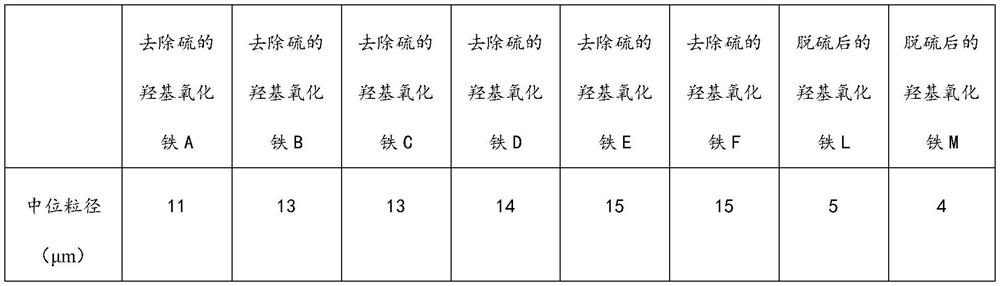
[0038] (2) The air-dried sulfur-containing iron oxyhydroxide and ethylbenzene were stirred and mixed at 90°C for 2 hours at a mass ratio of 1:10, and solid-liquid separation was performed to obtain a sulfur-containing solvent and sulfur-removed iron oxyhydroxide A;
[0039] (3) The sulfur-containing solvent is cooled and crystallized at a temperature of 5°C, and solid-liquid is separated to obtain sulfur A.
Embodiment 2
[0041] This embodiment provides a method for separating iron oxyhydroxide and sulfur, which includes the following steps:
[0042] (1) Dry the sulfur-containing iron oxyhydroxide at 90°C to a water content of 0.1 wt%;
[0043] (2) Stir and mix the air-dried sulfur-containing iron oxyhydroxide and methylcyclohexane at a mass ratio of 1:30 at 80°C for 1 hour, and separate the solid and liquid to obtain the first sulfur-containing solvent and the first iron oxyhydroxide;
[0044] (3) The first iron oxyhydroxide and methylcyclohexane were stirred and mixed at 80° C. for 1 hour at a mass ratio of 1:30, and solid-liquid separation was performed to obtain a second sulfur-containing solvent and a second iron oxyhydroxide;
[0045] (4) Mix the second iron oxyhydroxide and methylcyclohexane at a mass ratio of 1:30 at 80° C. for 1 h, and separate solid and liquid to obtain a third sulfur-containing solvent and sulfur-removed iron oxyhydroxide B;
[0046] (5) Combine the first, second and third sul...
Embodiment 3
[0048] This embodiment provides a method for separating iron oxyhydroxide and sulfur, which includes the following steps:
[0049] (1) Air-dry sulfur-containing iron oxyhydroxide at 25°C until its water content is 4wt%;
[0050] (2) Stir and mix the air-dried sulfur-containing iron oxyhydroxide and trichloroethane at a mass ratio of 1:7 at 50°C for 3 hours, and separate the solid-liquid to obtain the first sulfur-containing solvent and the first iron oxyhydroxide;
[0051] (3) The first ferric oxyhydroxide and trichloroethane were stirred and mixed at a mass ratio of 1:7 at 50°C for 3 hours, and solid-liquid separation was performed to obtain a second sulfur-containing solvent and a second ferric oxyhydroxide;
[0052] (4) Mix the second iron oxyhydroxide and trichloroethane at a mass ratio of 1:7 at 50°C for 3 hours, and separate the solid and liquid to obtain a third sulfur-containing solvent and sulfur-removed iron oxyhydroxide C;
[0053] (5) Combine the first, second and third sulf...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com