Method for removing sulfur on hydroxyl oxidize iron and application of sulfur-removed hydroxyl oxidize iron
A technology of iron oxyhydroxide and iron oxide, which is applied in the direction of iron oxide/iron hydroxide, chemical instruments and methods, separation methods, etc., can solve the problems such as the decline of desulfurization ability of iron oxyhydroxide, achieve easy recycling, ensure desulfurization performance, The effect of high solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This embodiment provides a method for removing sulfur on iron oxyhydroxide, comprising the steps of:
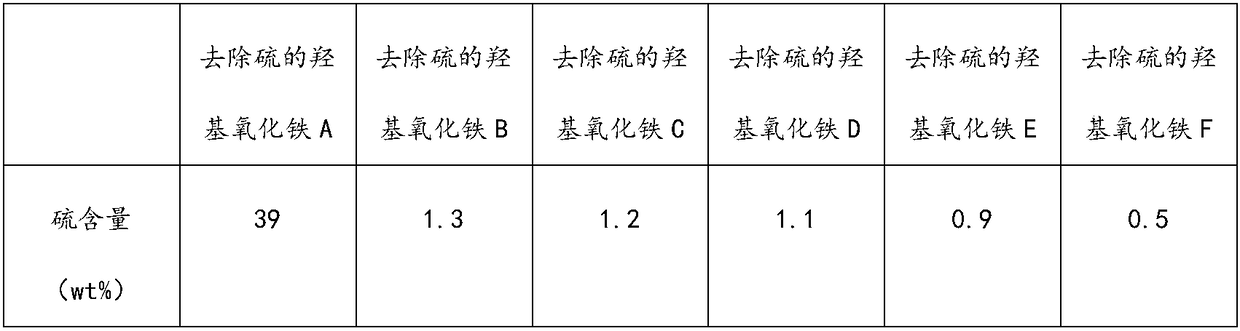
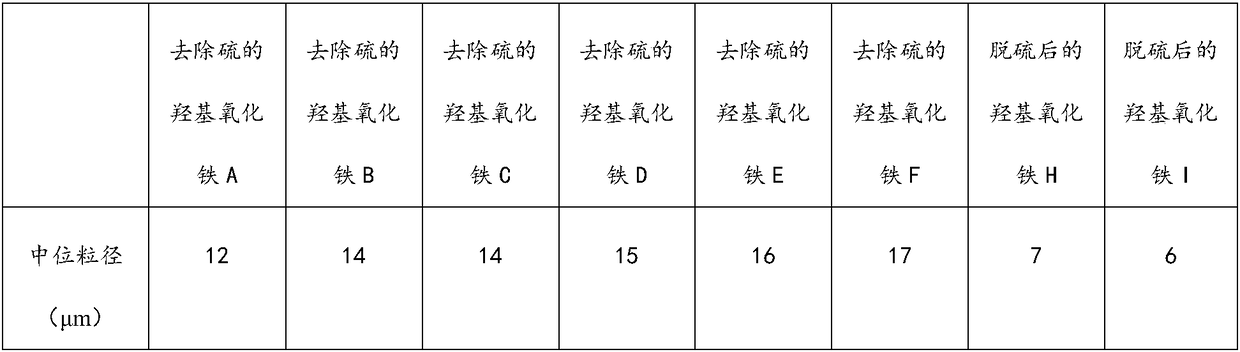
[0034] (1) At a temperature of 10° C., air-dry the sulfur-containing iron oxyhydroxide until its water content is 5 wt %;
[0035] (2) Stir and mix the air-dried sulfur-containing iron oxyhydroxide and toluene at 90° C. for 2 hours according to the mass ratio of 1:10, and separate the solid and liquid to obtain a sulfur-containing solvent and iron oxyhydroxide A from which sulfur has been removed;
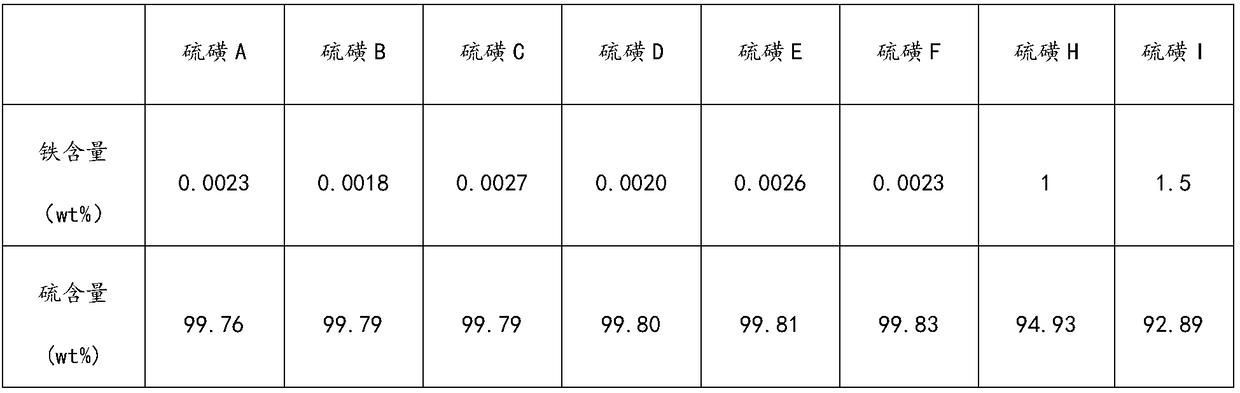
[0036] (3) The sulfur-containing solvent is cooled and crystallized at a temperature of 5° C., and solid-liquid separation is performed to obtain sulfur A (soluble sulfur).
Embodiment 2
[0038] This embodiment provides a method for removing sulfur on iron oxyhydroxide, comprising the steps of:
[0039] (1) drying sulfur-containing iron oxyhydroxide at 90°C until its water content is 0.1wt%;
[0040] (2) Stirring and mixing the air-dried sulfur-containing iron oxyhydroxide and toluene at 80° C. for 1 hour according to the mass ratio of 1:15, and separating the solid and liquid to obtain the first sulfur-containing solvent and the first iron oxyhydroxide;
[0041] (3) stirring and mixing the first iron oxyhydroxide and toluene at 80° C. for 1 hour according to the mass ratio of 1:15 to obtain the second sulfur-containing solvent and the second iron oxyhydroxide;
[0042] (4) Stir and mix the second iron oxyhydroxide and toluene at 80° C. for 1 hour according to the mass ratio of 1:15, and separate the solid and liquid to obtain the third sulfur-containing solvent and iron oxyhydroxide B from which sulfur has been removed;
[0043] (5) Combine the first, second ...
Embodiment 3
[0045] This embodiment provides a method for removing sulfur on iron oxyhydroxide, comprising the steps of:
[0046] (1) air-drying sulfur-containing iron oxyhydroxide at 20°C until its water content is 0.2wt%;
[0047] (2) Stirring and mixing the air-dried sulfur-containing iron oxyhydroxide and toluene at 50° C. for 3 hours according to the mass ratio of 1:7, and separating the solid and liquid to obtain the first sulfur-containing solvent and the first iron oxyhydroxide;
[0048] (3) Stir and mix the first iron oxyhydroxide and toluene at 50°C for 3 hours according to the mass ratio of 1:7, and separate the solid and liquid to obtain the second sulfur-containing solvent and the second iron oxyhydroxide;
[0049] (4) Stir and mix the second iron oxyhydroxide and toluene at 50°C for 3 hours according to the mass ratio of 1:7, and separate the solid and liquid to obtain the third sulfur-containing solvent and iron oxyhydroxide C from which sulfur has been removed;
[0050] (5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com