Hydrophobic Modified Polyethyleneimine Carbon Dioxide Absorbent and Its Application
A technology of polyethylenimine and absorbent, applied in the direction of reagents, chemical instruments and methods, separation methods, etc., can solve problems such as difficult diffusion and reduced CO2 absorption efficiency, and achieve the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-20
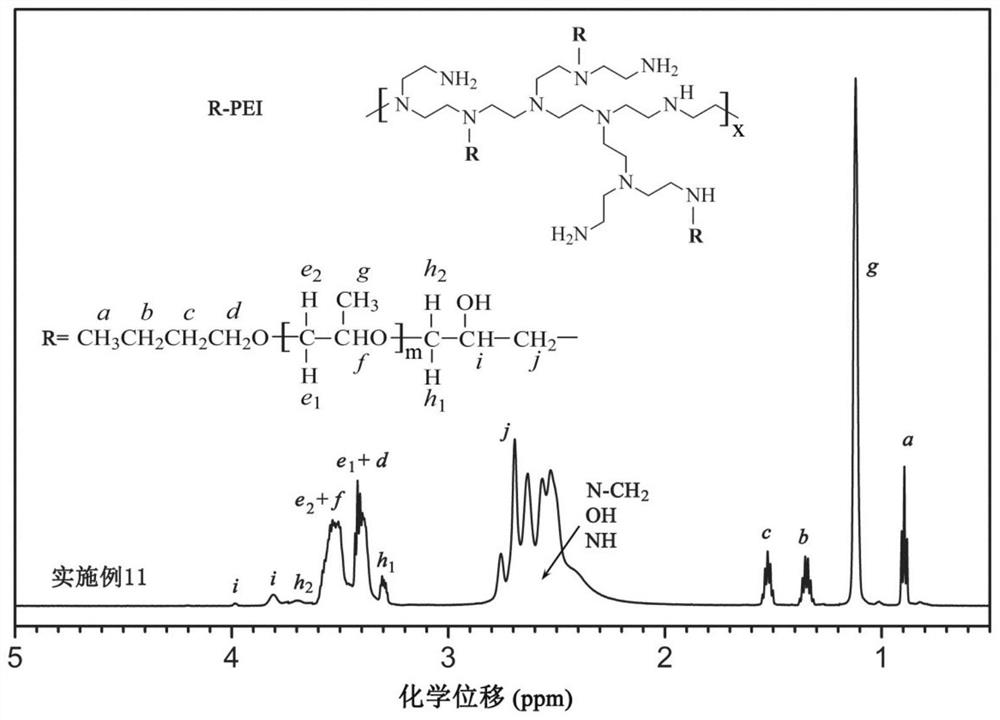
[0071] This group is an example of generating hydrophobically modified polyethyleneimine through the ring-opening reaction of the epoxy group of polyether glycidyl ether and the amine group in polyethyleneimine. The structure of polyether glycidyl ether is:
[0072]
[0073] (The parameters such as m and n are shown in Table 1). The linking group between the hydrophobic side chain and the main chain of the hydrophobically modified polyethyleneimine obtained by this group is Q1:
[0074] It should be pointed out that the mole fraction of PEI in Table 1 refers to the mole fraction of its repeating unit, that is, the mole fraction of nitrogen in PEI. The repeating units m of the polyether glycidyl ethers used may have fractional average values due to the molecular weight distribution of the polymers.
[0075] Table 1
[0076]
[0077]
[0078]
[0079]The above-mentioned hydrophobically modified polyethyleneimine is prepared by the following method: polyethylen...
Embodiment 21-30
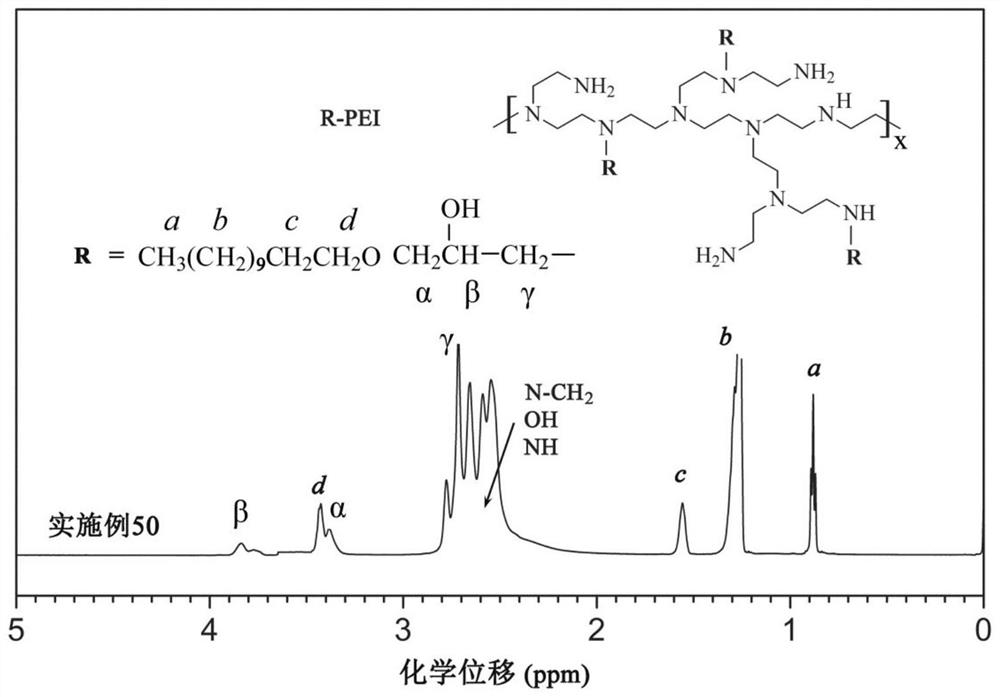
[0082] This group of embodiments first synthesizes polyether hydrophobic chain halogenated hydrocarbons, and its structural formula is (see Table 2 for parameters such as m and n) It should be pointed out that the repeating unit of the structural formula here is a repeating unit of polypropylene glycol (PPG), which can also be replaced by a repeating unit of polyoxetane (PTG) or polytetrahydrofuran ether (PTMG). The type of polyether side chain See Table 2.
[0083] Secondly, the polyether hydrophobic chain halogenated hydrocarbon and the amine group of branched polyethyleneimine (molecular weight 25000) are alkylated to obtain hydrophobically modified polyethyleneimine, and the connecting group between the main chain and the side chain For Q2: (corresponding to iodohydrocarbon, embodiment 21-25) or Q3: (corresponding brominated hydrocarbon, embodiment 26-30).
[0084] Table 2
[0085]
[0086] The specific synthesis process is as follows: For Examples 21-25, take 1 ...
Embodiment 31-40
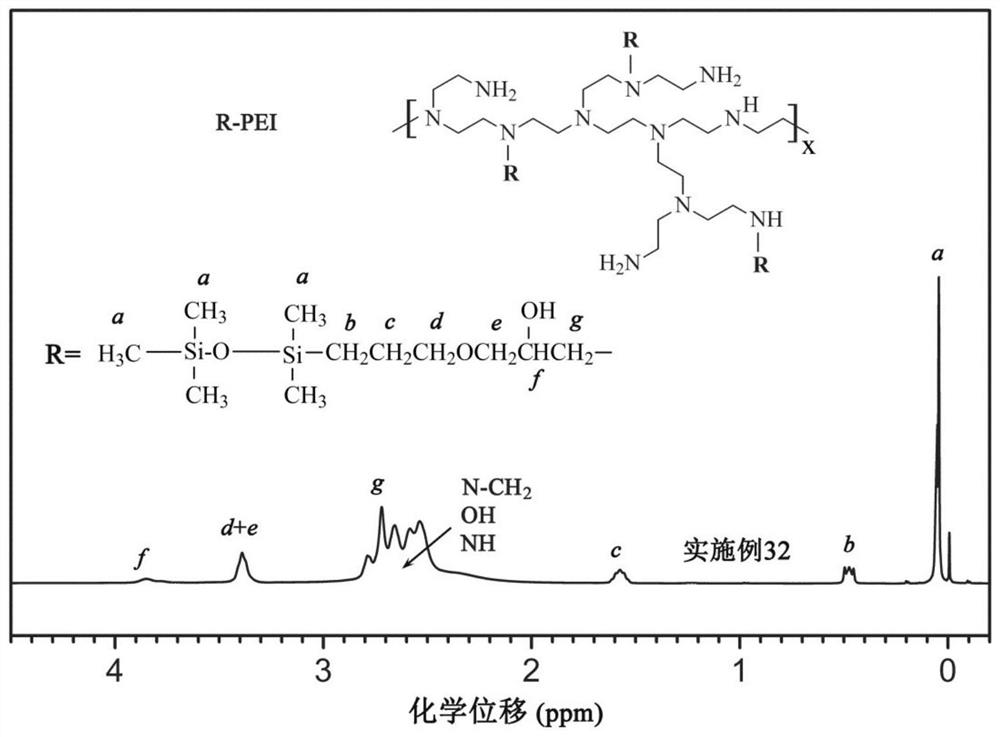
[0090] This group of embodiments first synthesizes glycidyl ether of silane (m=0) or siloxane (m is greater than or equal to 1), and its structural formula is (see Table 3 for parameters such as m):
[0091] (abbreviated as PTMS, see Table 3);
[0092] Secondly, carry out alkylation reaction with the synthesized silane or siloxane glycidyl ether and the amine group of polyethyleneimine to obtain hydrophobically modified polyethyleneimine, and the connecting group between the main chain and the side chain is T :
[0093] table 3
[0094]
[0095] For each group of embodiments, the corresponding PTMS (see Table 3) is first synthesized, and the synthetic raw materials are trimethylsilane (m=0) or siloxane (m≠0) and allyl glycidyl ether terminated by silicon hydrogen , the molar parts of the two are respectively 1 molar part and 1.05 molar parts, and the two raw materials are dissolved in anhydrous isopropanol, so that the mass content of the raw materials is 30%, blowing...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com