A kind of electroluminescent material based on anthracene derivative and its preparation method and application
A technology of electroluminescent materials and anthracene derivatives, applied in the directions of luminescent materials, chemical instruments and methods, circuits, etc., can solve the problems of difficult to form amorphous films, reduce the optoelectronic properties of devices, restrict development, etc., and achieve high-efficiency and stable device performance, Improve solubility and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] Preparation of an anthracene derivative monomer
[0053] Preparation of ethylthiophene-3-carboxylate
[0054] In a 500mL three-necked flask, thiophene-3-carboxylic acid (12.8g, 0.1mol) was dissolved in 200mL of methanol, and 20mL of concentrated sulfuric acid (98wt%) was added dropwise to the reaction solution, and after stirring at room temperature for 12 hours, the reaction was stopped. The reaction was quenched with water, extracted with dichloromethane and dried with anhydrous magnesium sulfate. After the solution was concentrated, a yellow liquid was obtained, which was purified by silica gel column chromatography. The mixed solvent of petroleum ether / dichloromethane (5 / 1, v / v ) is the eluting agent, and the productive rate is 73%. 1 H NMR, 13 CNMR, MS and elemental analysis results show that the obtained compound is the target product, and the chemical reaction equation of the preparation process is as follows:
[0055]
[0056] Preparation of ethyl 2-(tribu...
Embodiment 1
[0072] The preparation of embodiment 1 compound B1
[0073] Under argon atmosphere, in a 100mL three-necked flask, add 2,9-dibromo-7,7,14,14-tetrabutyl-7,14-dihydroperylene[1,2-b:7,8 -b']dithiophene (1.80g, 2.4mol), bis(9,9-dimethyl-9H-fluoren-2-yl)amine (2.02g, 5.0mmol), sodium tert-butylate (1.84g, 19.2mmol), palladium acetate (27mg, 0.12mmol) and 50ml toluene. Heat and stir to 85°C, add 0.12ml of tri-tert-butylphosphine in toluene (0.24mmol, 2mol / L), and react for 12h. After the reaction was stopped, the solvent was concentrated, and the crude product was purified by column chromatography using a mixed solvent of petroleum ether and dichloromethane (2 / 1, v / v) as the eluent to obtain a green solid, which was named compound B1. 1 H NMR, 13 CNMR, MS and elemental analysis results show that the obtained compound is the target product, and the chemical reaction equation of the preparation process is as follows:
[0074]
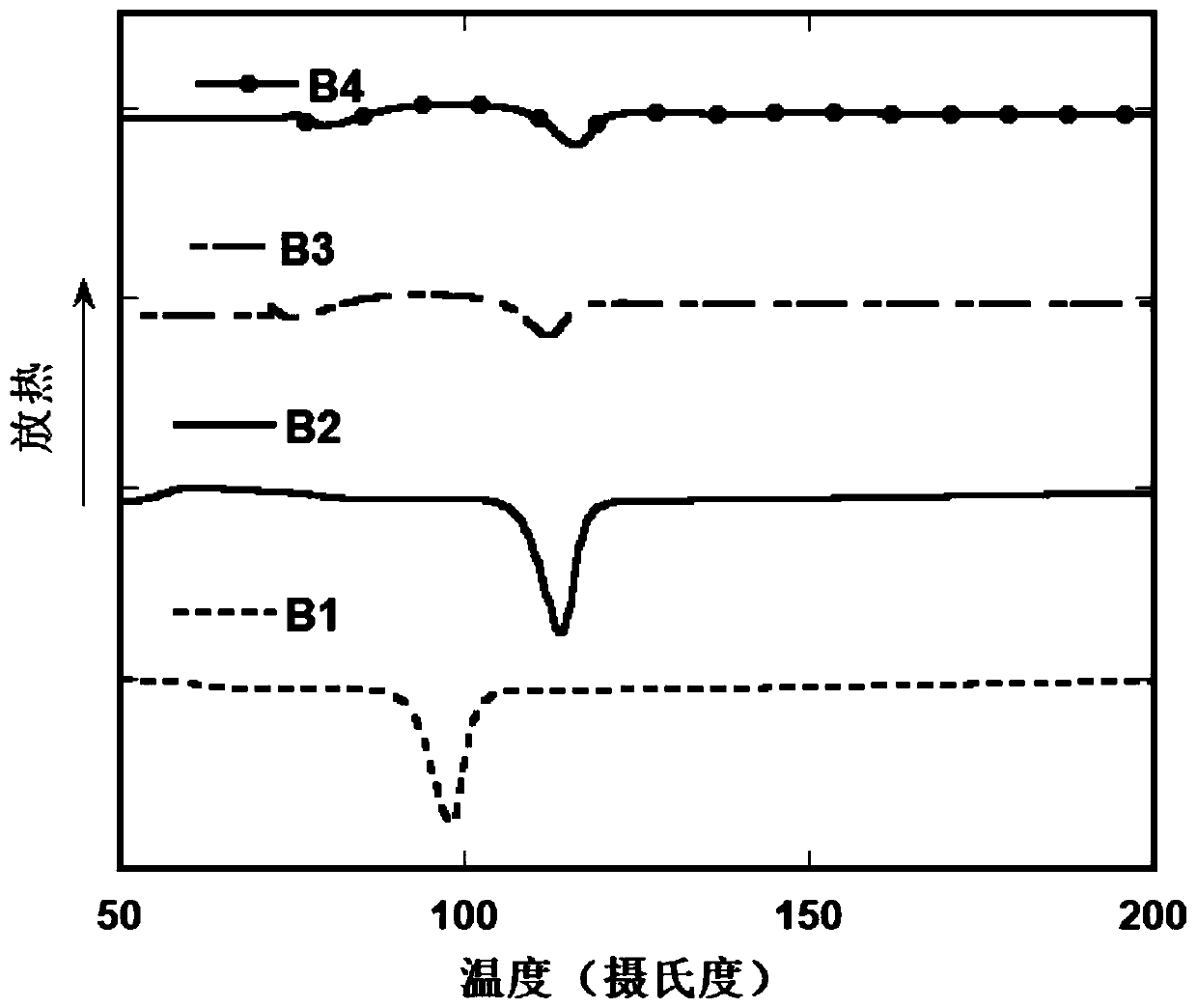
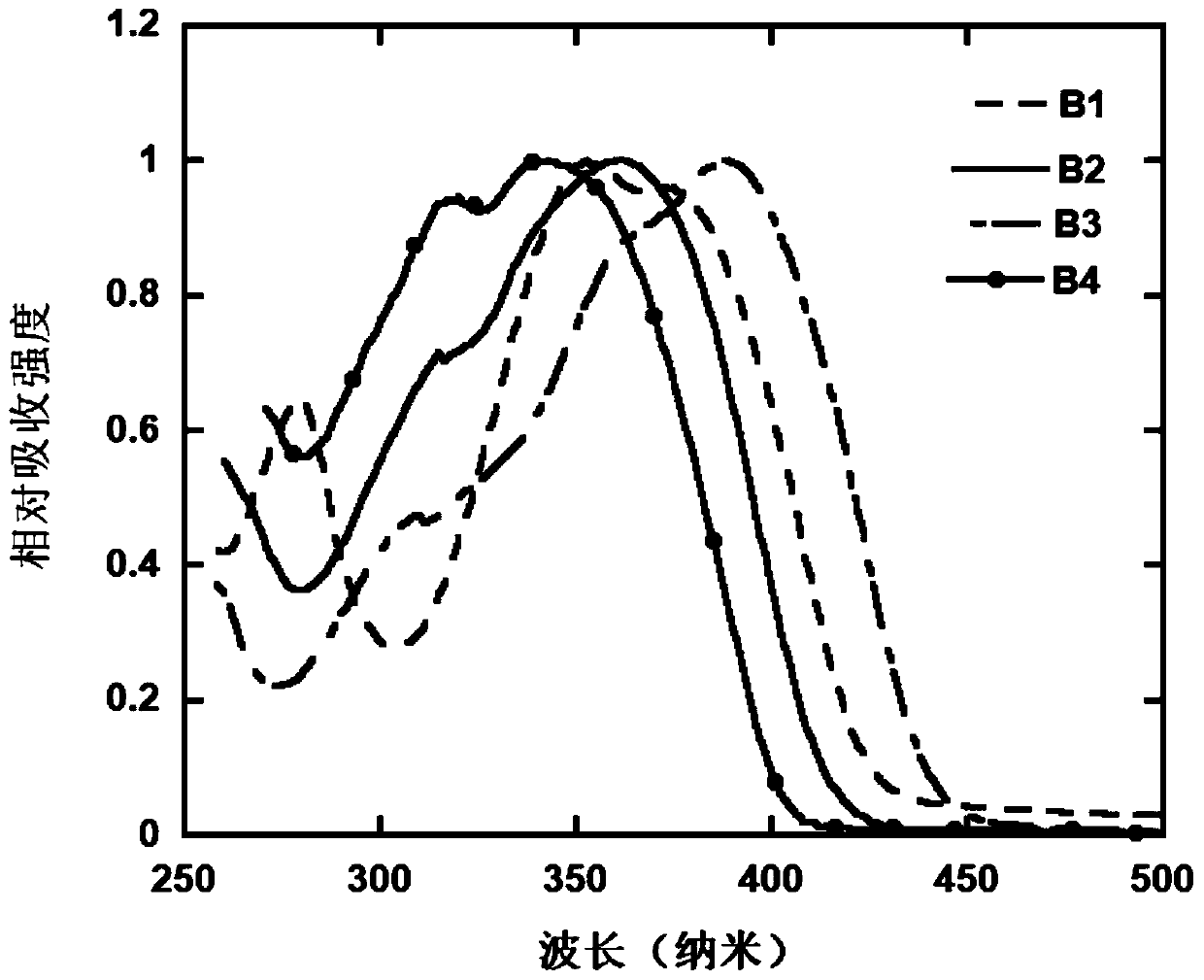
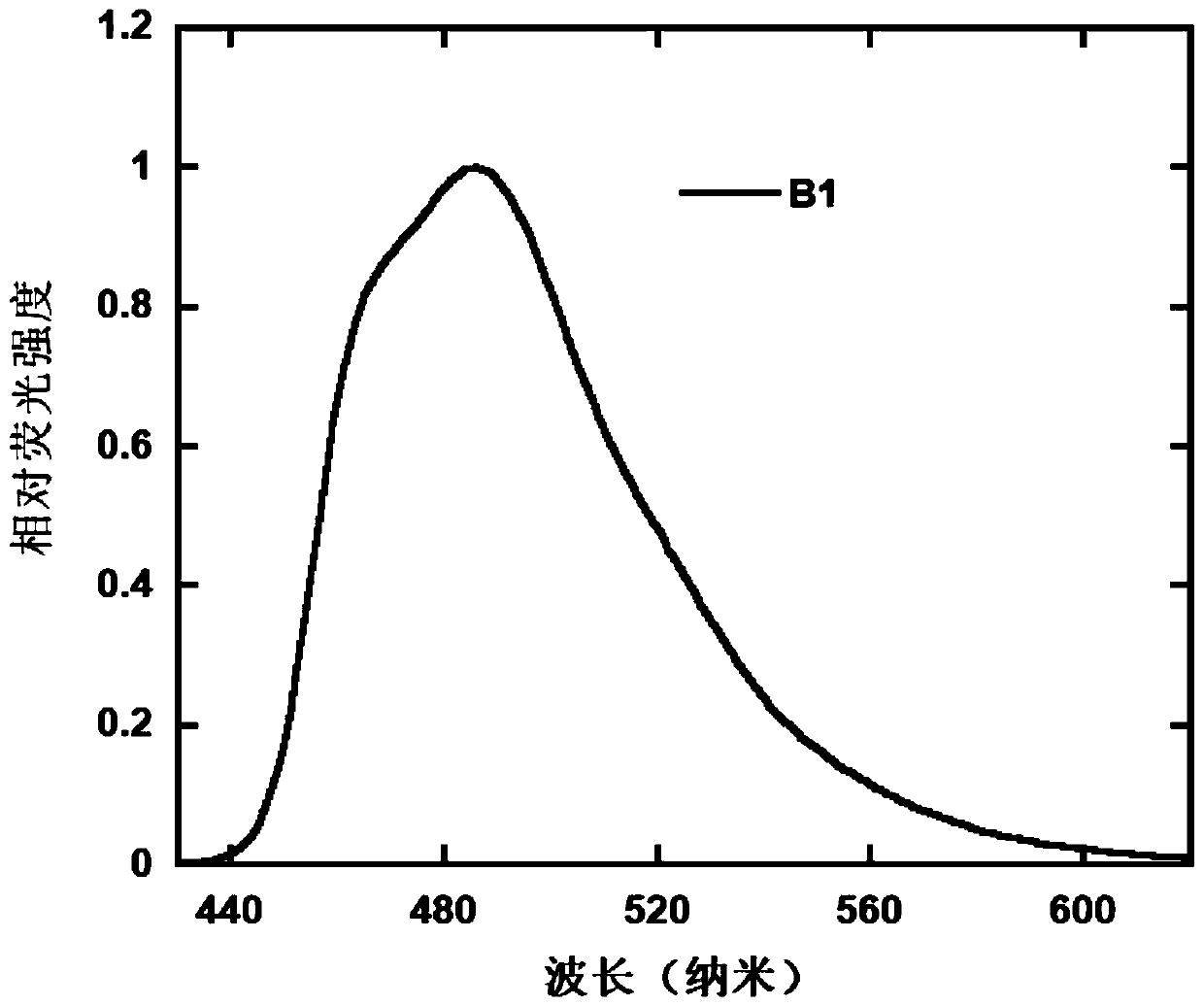
[0075] The differential scanning calorimetry (DSC)...
Embodiment 2
[0078] The synthesis of embodiment 2 compound B2
[0079]Under argon atmosphere, in a 100mL three-necked flask, add 2,9-dibromo-7,7,14,14-tetrabutyl-7,14-dihydroperylene[1,2-b:7,8 -b']dithiophene (1.80g, 2.4mol), N-[1,1-biphenyl]-4-yl-9,9-dimethyl-9H-fluorene-3-amine (1.81g, 5.0mmol ), sodium tert-butylate (1.84g, 19.2mmol), palladium acetate (27mg, 0.12mmol) and 50ml of toluene. Heat and stir to 85°C, add 0.12ml of tri-tert-butylphosphine in toluene (0.24mmol, 2mol / L), and react for 12h. After stopping the reaction, the solvent was concentrated, and the crude product was purified by column chromatography, using a mixed solvent of petroleum ether and dichloromethane (3 / 1, v / v) as the eluent, and finally a green solid was obtained, which was named compound B2. 1 H NMR, 13 C NMR, MS and elemental analysis results show that the compound obtained is the target product, and the chemical reaction equation of the preparation process is as follows:
[0080]
[0081] The differe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com