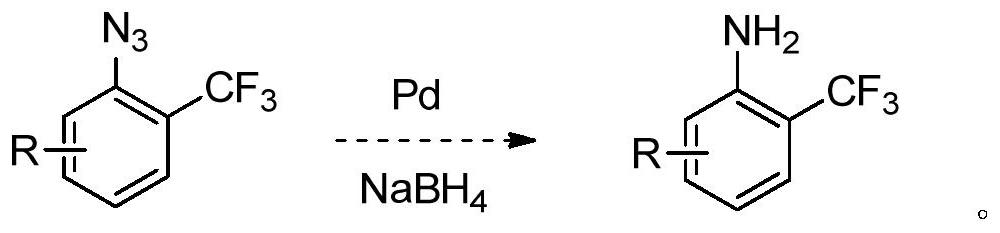
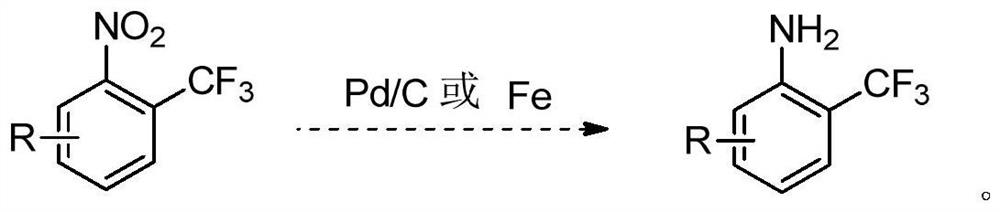
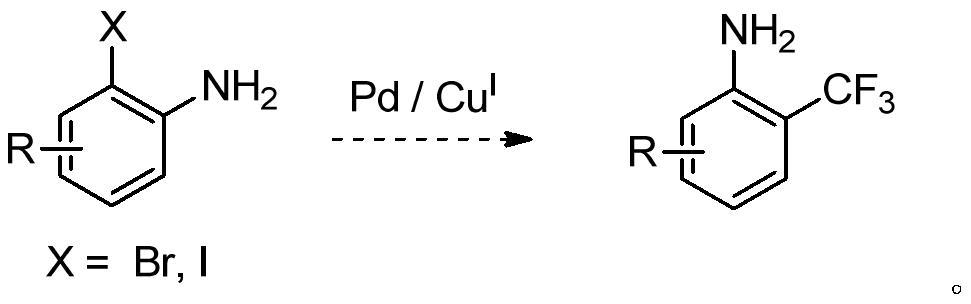
A kind of synthetic method of o-aminobenzobenzotrifluoride and derivatives thereof
A technology of o-aminotrifluorotoluene and aminotrifluorotoluene is applied in the field of synthesis of o-aminotrifluorotoluene and derivatives thereof, and can solve the problems such as being unsuitable for industrial mass production, difficulty in synthesizing azide compounds, increasing production costs, and the like, Achieve the effect of convenient production, easy purification, and less waste.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-7
[0046] The target compound o-aminobenzotrifluoride has the structural formula:
[0047]
[0048] The reaction equation is:
[0049]
[0050] Synthesized by:
[0051] At room temperature, in a dry 50mL three-necked reaction flask, add a magneton, and then add 20mL of anhydrous acetonitrile, then add the oxidant (1.5mmol) to anhydrous acetonitrile under stirring conditions, and then add trifluoroform Trimethylsilane (213mg, 1.5mmol), then added anhydrous potassium acetate (147mg, 1.5mmol), then heated the reaction solution to 80°C, and dissolved aniline (93mg, 1.0mmol) in 5mL of anhydrous acetonitrile , and then through a constant pressure dropping funnel, slowly add the aniline solution dropwise to the above reaction solution at 80° C. for about 1 hour. After the dropwise addition was completed, the reaction was carried out at 80° C. for 12 hours. The reaction solution was lowered to room temperature, the solid was filtered off, and then the reaction solution was spin-...
Embodiment 8-11
[0059] The target compound o-aminobenzotrifluoride has the structural formula:
[0060]
[0061] The reaction equation is:
[0062]
[0063] Synthesized by:
[0064] At room temperature, in a dry 50mL three-necked reaction flask, add a magnet, and then add 20mL of anhydrous acetonitrile, and then stir the oxidant 1-acetoxy-1,2-phenyliodide-3- (1H)-ketone (460mg, 1.5mmol) was added to anhydrous acetonitrile, then trifluoromethyltrimethylsilane (213mg, 1.5mmol) was added, and alkali (1.5mmol) was added, then the reaction solution was heated to 80 ℃, dissolve aniline (93mg, 1.0mmol) in 5mL of anhydrous acetonitrile, and then slowly add the aniline solution dropwise to the above reaction solution at 80℃ through a constant pressure dropping funnel for about 1 hour. After the dropwise addition was completed, the reaction was carried out at 80° C. for 12 hours. The reaction solution was lowered to room temperature, the solid was filtered off, and then the reaction solution w...
Embodiment 12-15
[0070] The target compound o-aminobenzotrifluoride has the structural formula:
[0071]
[0072] The reaction equation is:
[0073]
[0074] Synthesized by:
[0075] At room temperature, in a dry 50mL three-necked reaction flask, add a magneton, and then add 20mL of solvent, and then stir the oxidant 1-acetoxy-1,2-phenyliodyl-3-(1H )-ketone (460mg, 1.5mmol) was added to the solvent, then trifluoromethyltrimethylsilane (213mg, 1.5mmol) was added, then anhydrous potassium acetate (147mg, 1.5mmol) was added, and the reaction solution was heated to Dissolve aniline (93 mg, 1.0 mmol) in 5 mL of solvent at 80°C, and slowly add the aniline solution dropwise to the above reaction solution at 80°C through a constant pressure dropping funnel for about 1 hour. After the dropwise addition was completed, the reaction was carried out at 80° C. for 12 hours. The reaction solution was lowered to room temperature, the solid was filtered off, and then the reaction solution was spin-dri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com