Measurement method of optical purity of 4-(3H)-quinazolinone compound alkaloid
A determination method and quinazolinone technology are applied in the field of optical purity of 4-quinazolinone alkaloids, and achieve the effects of high basic research value, social and economic benefits, simple operation and high precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
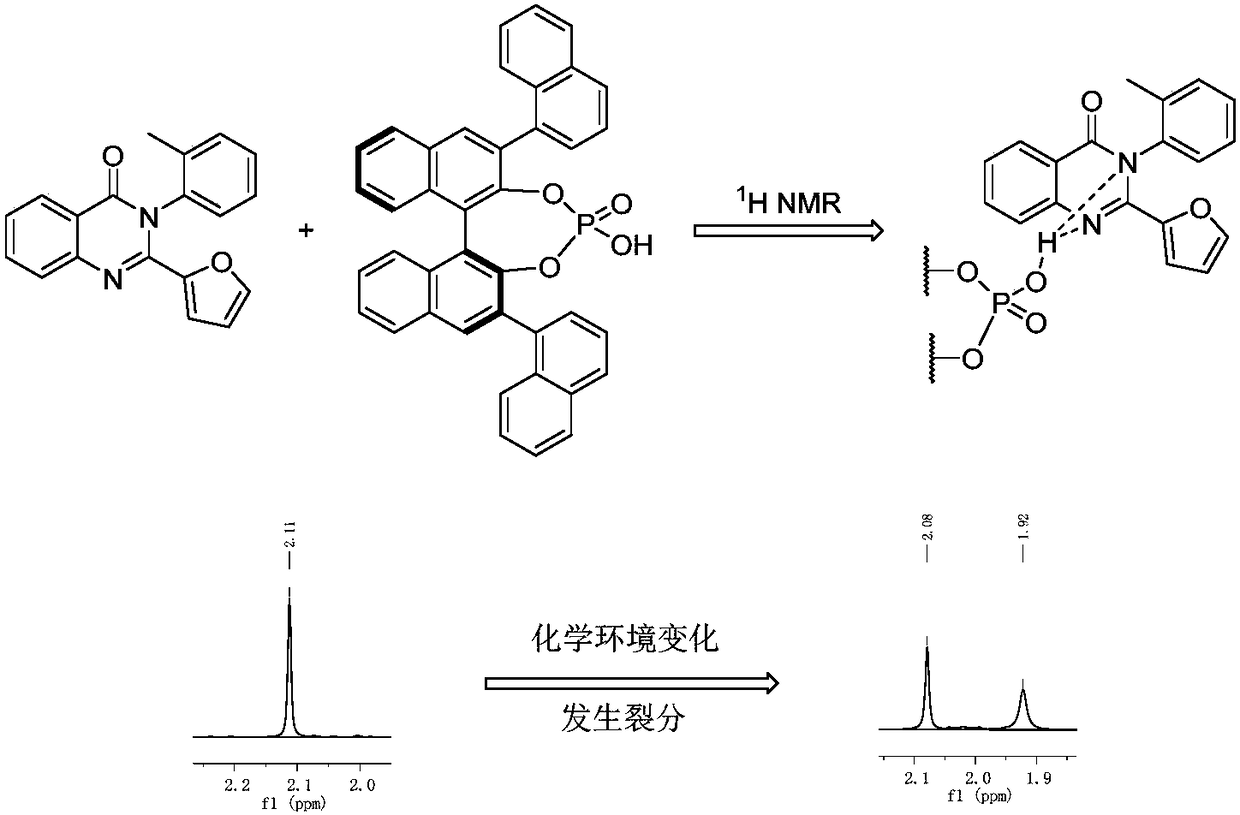
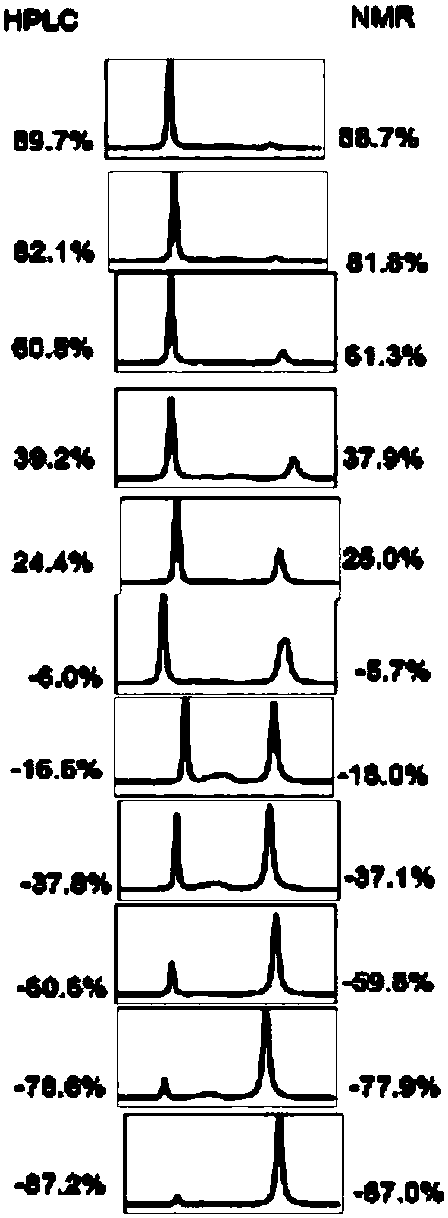
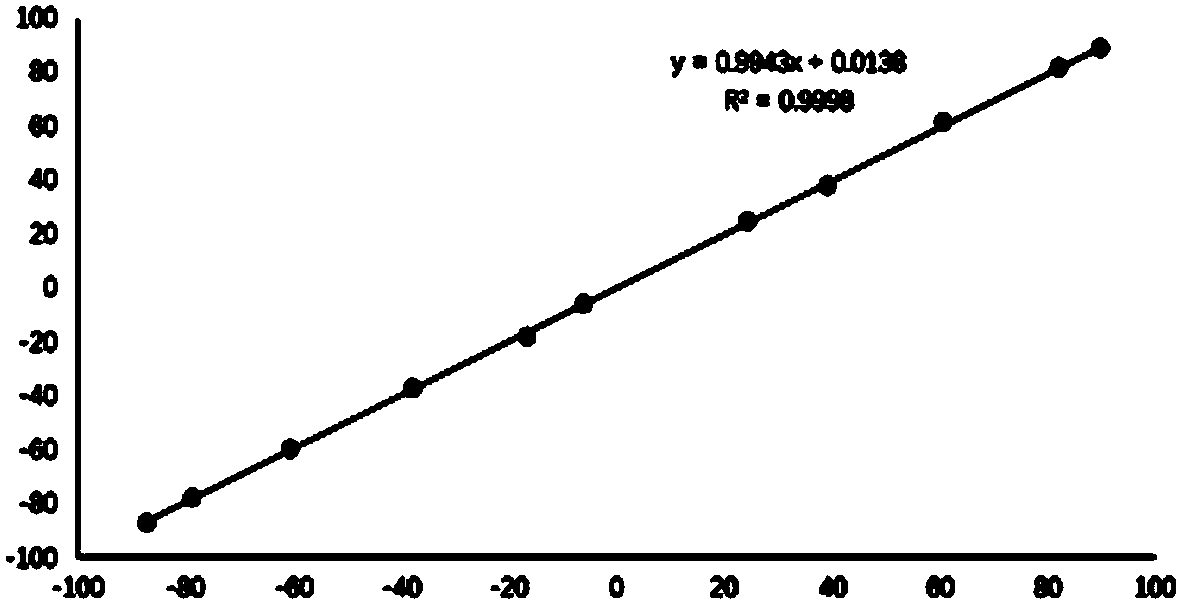
[0026] R 1 for hydrogen, R 2 for hydrogen, R 3 is a furan group, R 4 The substance ratio of methyl 4-(3H) quinazolinone 1a to chiral phosphoric acid recognition agent is 1.0:1.0, substrate 4-(3H) quinazolinone 3.0mg (0.1mmol), ( S)-3,3'-bis(1-naphthyl)-1,1'-binaphthol phosphate 6.0 mg (0.1 mmol); the deuterated solvent is deuterated chloroform 0.6 mL. At room temperature, add (S)-3,3'-bis(1-naphthyl)-1,1'-binaphthol phosphate and 4-(3H)quinazolinone compounds into the NMR tube, add After deuterated chloroform was dissolved and mixed evenly, the H-NMR spectrum was detected. The optical purity of the 4-(3H)quinazolinone compound is determined by calculating the chemical shift shift and ratio of the characteristic peaks of the diastereoisomers. Here, the H NMR spectrum of the methyl group of the 4-(3H) quinazolinone compound is selected as the characteristic peak for comparison, and the chemical shift shift is ΔΔδ=0.16ppm. The specific structure and NMR characterization of ...
Embodiment 2
[0028] Substrate 4-(3H) quinazolinone 3.0mg (0.1mmol), (S)-3,3'-bis(1-naphthyl)-1,1'-binaphthol phosphate 6.0mg (0.1mmol ); deuterated solvent is deuterated methanol 0.6mL.
[0029] The rest are the same as in Example 1, and the chemical shift shift is ΔΔδ=0.01ppm.
Embodiment 3
[0031] Substrate 4-(3H) quinazolinone 3.0mg (0.1mmol), (S)-3,3'-bis(1-naphthyl)-1,1'-binaphthol phosphate 6.0mg (0.1mmol ); deuterated solvent is deuterated acetone 0.6mL.
[0032] The rest are the same as in Example 1, and the chemical shift shift is ΔΔδ=0.03ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap