Pharmaceutical composite preparation
A tablet, pharmaceutical technology, applied in the field of preparation of the tablet, can solve the problems of reducing drug efficacy, poor inherent solubility, low water solubility, etc., to achieve the treatment or prevention of high blood pressure, good tablet formation and production efficiency, and good stability The effect of sex and solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: the preparation of bilayer tablet
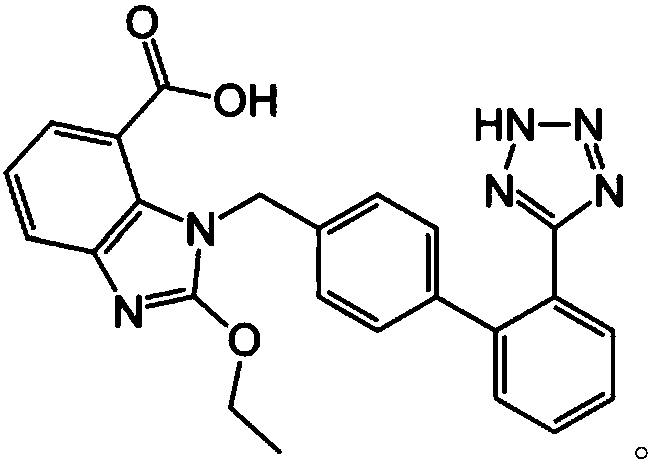
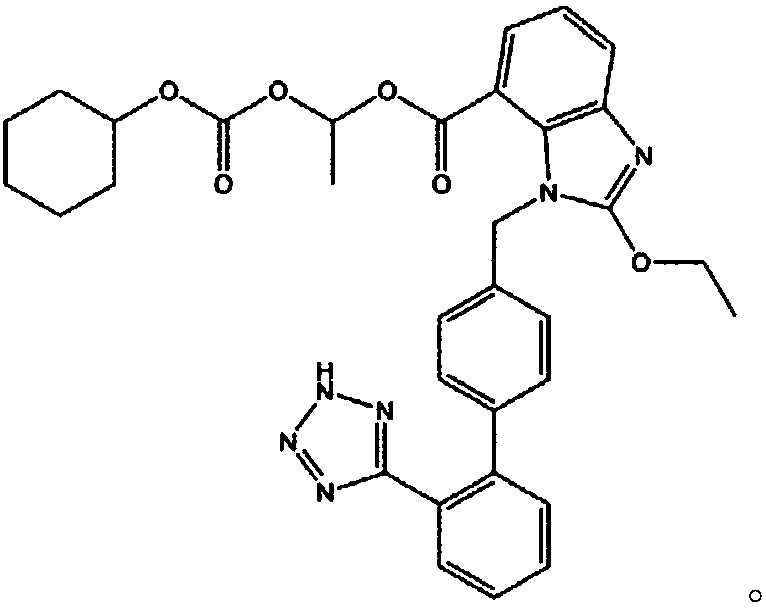
[0062] 6 g of hydroxypropylcellulose and 3 g of polyethylene glycol 15-hydroxystearate were dissolved in purified water and ethanol to prepare a binding solution (1). 16 g of candesartan cilexetil, 65 g of microcrystalline cellulose, and 10 g of pregelatinized starch were uniformly mixed in a high-speed mixer to prepare a mixture (1). The binding solution (1) is added to the mixture (1), granulated and then dried. Sieved granules (1) were obtained by sieving with a 20-mesh sieve in a semi-dry state and with a 30-mesh sieve in a dry state.
[0063] 79 g of silicified microcrystalline cellulose were added to the sieved granules (1 ) and mixed in a double cone mixer. Then, 1 g of magnesium stearate was added to obtain a lubricated mixture (1).
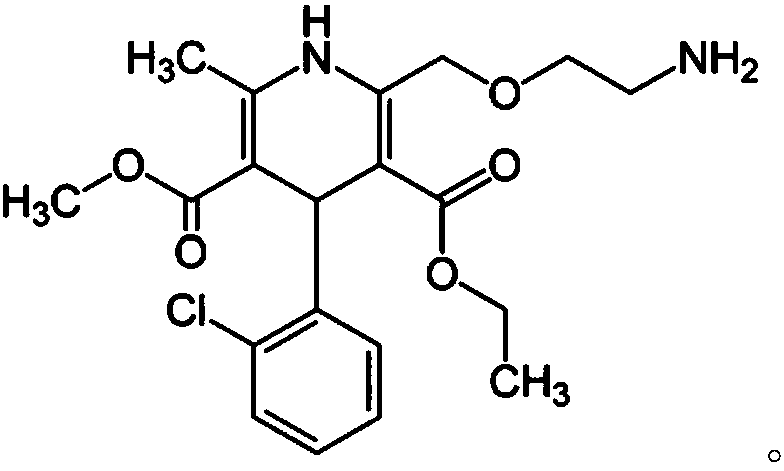
[0064] 5 g of hydroxypropylcellulose was dissolved in purified water and ethanol to prepare a binding solution (2). 13.87 g of amlodipine besylate, 71.13 g of microcrystalline c...
Embodiment 2
[0068] Embodiment 2: the preparation of bilayer tablet
[0069] 6 g of hydroxypropylcellulose and 3 g of polyethylene glycol 15-hydroxystearate were dissolved in purified water and ethanol to prepare a binding solution (1). 16 g of candesartan cilexetil, 65 g of microcrystalline cellulose, and 10 g of pregelatinized starch were uniformly mixed in a high-speed mixer to prepare a mixture (1). The binding solution (1) is added to the mixture (1), granulated and then dried. Sieved granules (1) were obtained by sieving with a 20-mesh sieve in a semi-dry state and with a 30-mesh sieve in a dry state.
[0070] 79 g of silicified microcrystalline cellulose were added to the sieved granules (1 ) and mixed in a double cone mixer. Then, 1 g of magnesium stearate was added to obtain a lubricated mixture (1).
[0071] 2.5 g of hydroxypropylcellulose was dissolved in purified water and ethanol to prepare a binding solution (2). 6.935 g of amlodipine besylate, 35.565 g of microcrystallin...
Embodiment 3
[0075] Embodiment 3: the preparation of bilayer tablet
[0076] 3 g of hydroxypropylcellulose and 1.5 g of polyethylene glycol 15-hydroxystearate were dissolved in purified water and ethanol to prepare a binding solution (1). 8 g of candesartan cilexetil, 32.5 g of microcrystalline cellulose, and 5 g of pregelatinized starch were uniformly mixed in a high-speed mixer to prepare mixture (1). The binding solution (1) is added to the mixture (1), granulated and then dried. Sieved granules (1) were obtained by sieving with a 20-mesh sieve in a semi-dry state and with a 30-mesh sieve in a dry state.
[0077] 39.5 g of silicified microcrystalline cellulose were added to the sieved granules (1 ) and mixed in a double cone mixer. Then, 0.5 g of magnesium stearate was added to obtain a lubricated mixture (1).
[0078] 2.5 g of hydroxypropylcellulose was dissolved in purified water and ethanol to prepare a binding solution (2). 6.935 g of amlodipine besylate, 35.565 g of microcrysta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com