Treatment of myelosuppression
一种骨髓抑制、治疗方案的技术,应用在治疗、放射疗法、含有效成分的医用配制品等方向,能够解决降低治疗剂量、治疗方案副作用、延迟等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
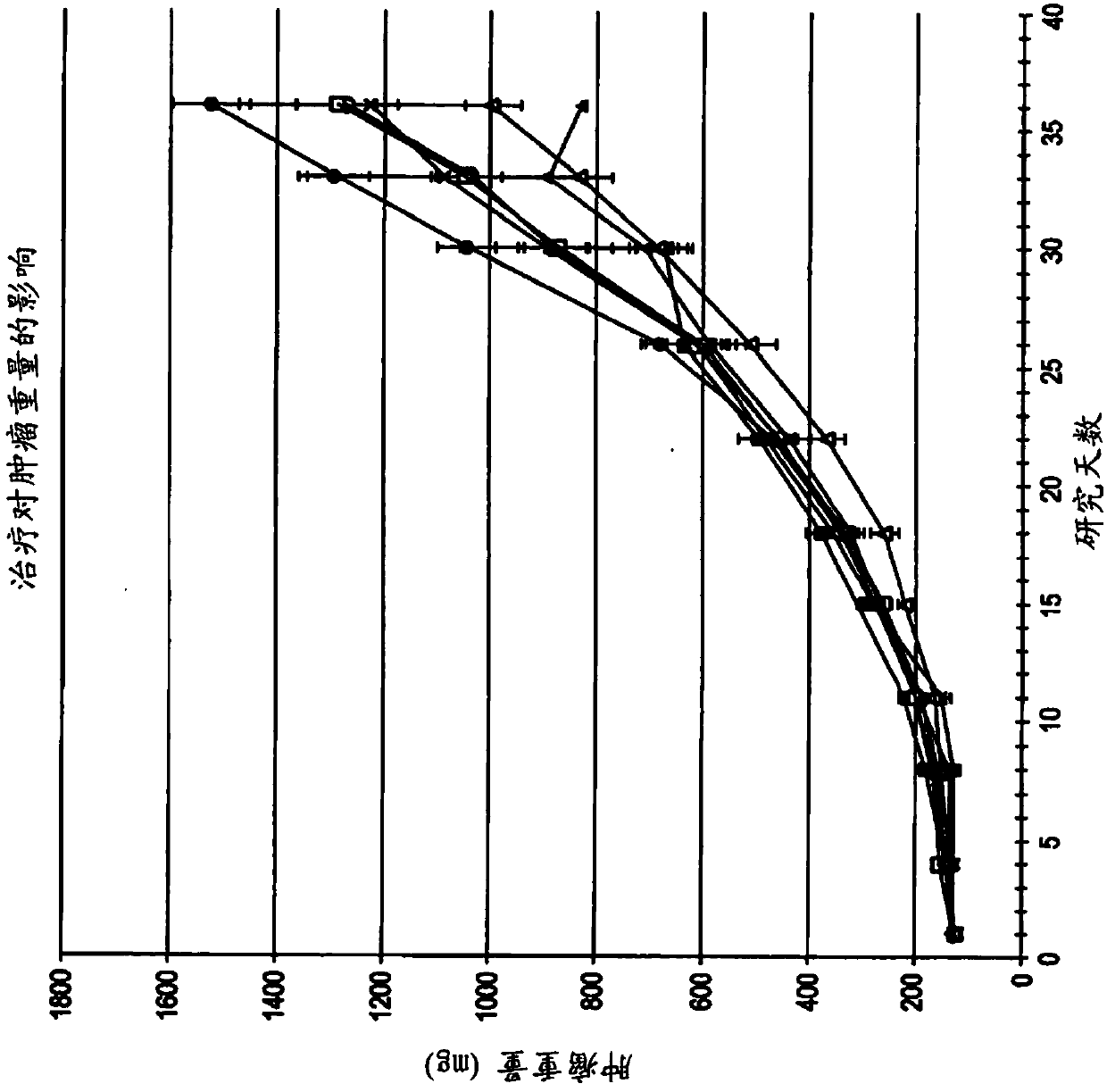
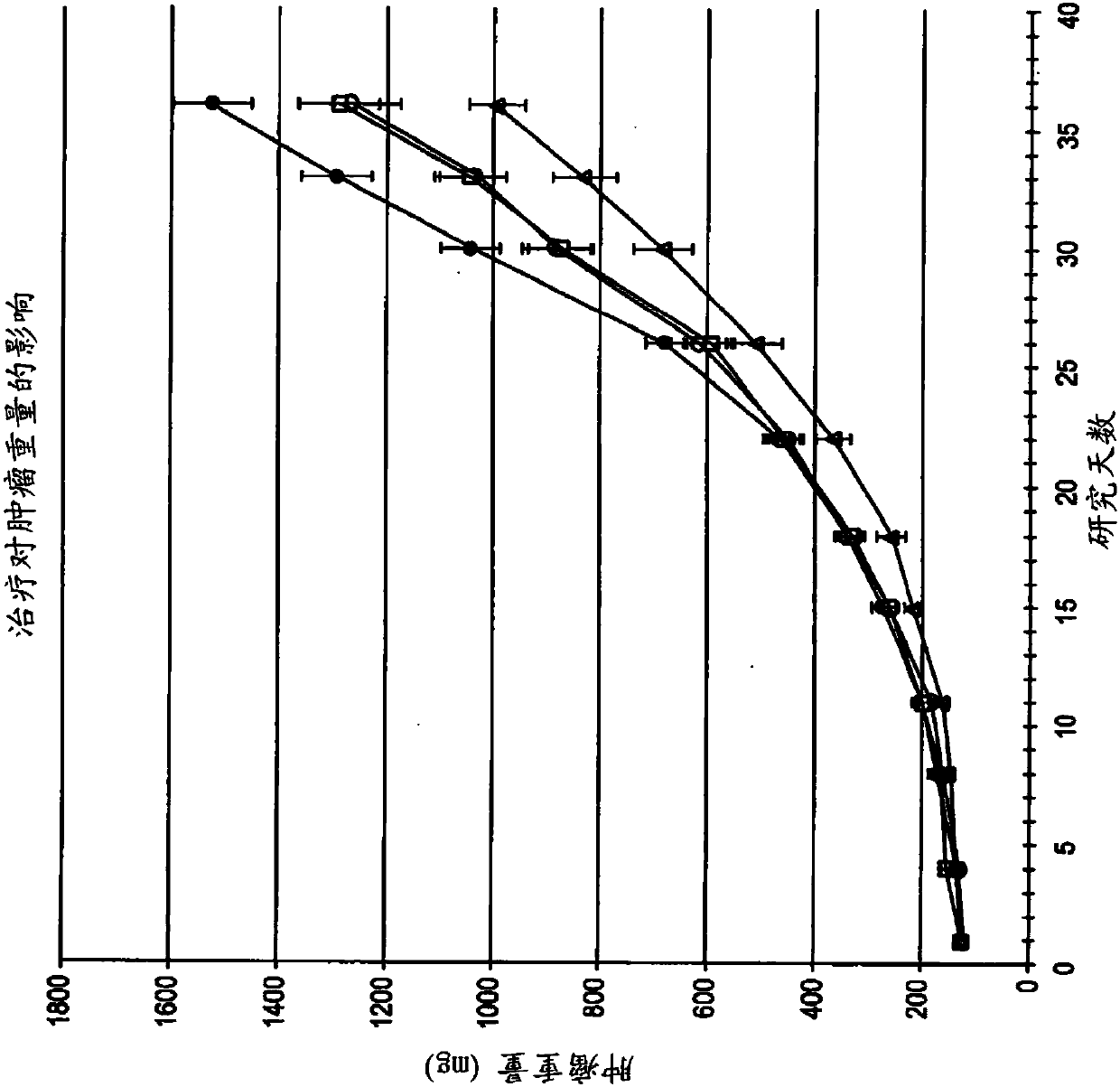
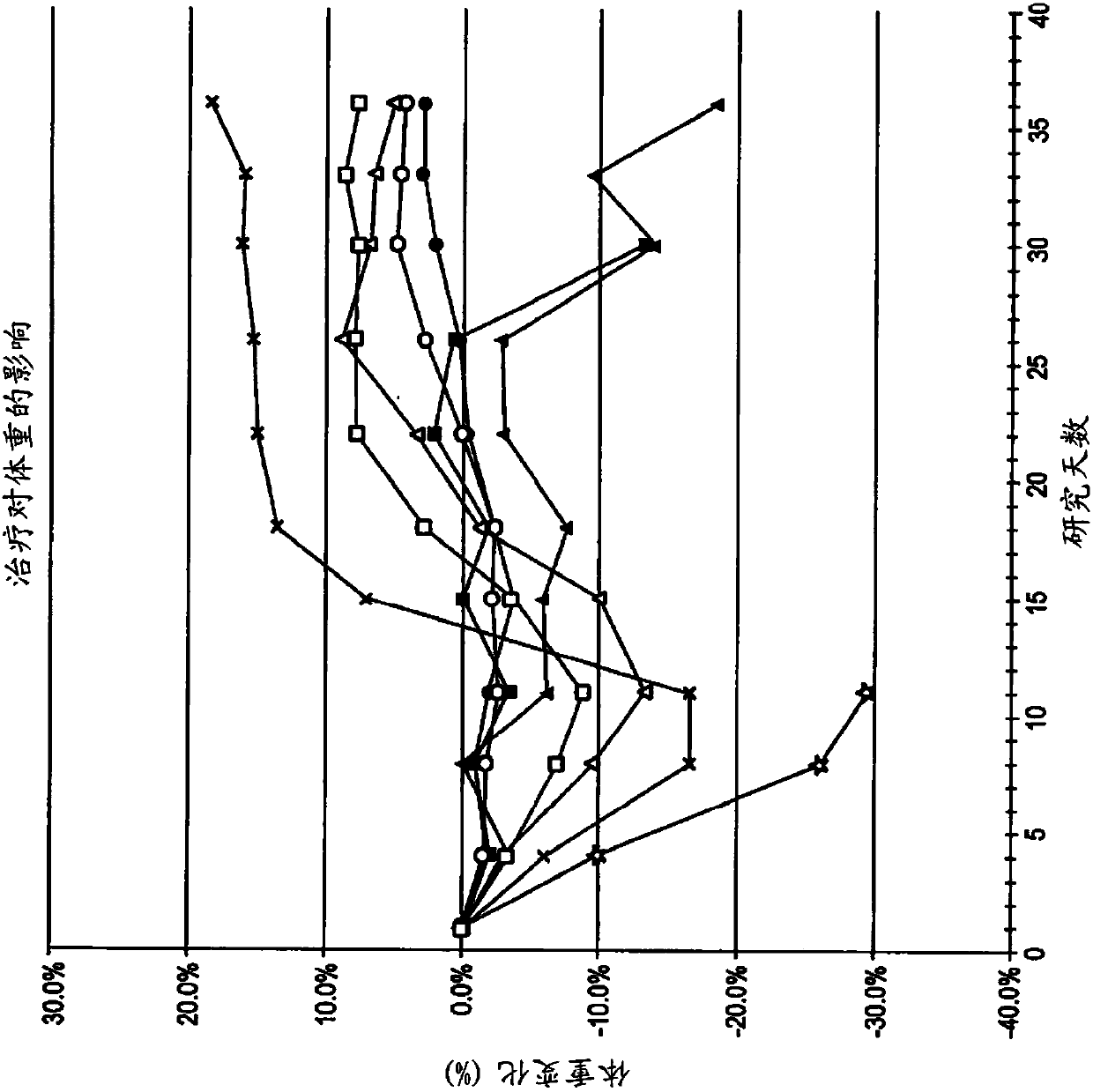
[0249] 6.2 Example 2: ODSH, a heparanoid that interacts with PF4, enhances the efficacy of carboplatin in a mouse xenograft model of human ovarian cancer
[0250] This example demonstrates that adjuvant administration of ODSH enhances the efficacy of carboplatin against human ovarian tumors grown as xenografts in athymic nude mice.
[0251] Materials & Methods ODSH (50 mg / mL stock concentration) was prepared by Pyramid Laboratories, Inc. and stored at room temperature until use. As described further below, ODSH can be administered either intravenously (IV) at a dose of 48 mg / kg and a volume of 10 mL / kg or subcutaneously (SC) at a dose of 24 mg / kg and a volume of 5 mL / kg. Carboplatin obtained from clinical suppliers was stored at 4°C until use. Carboplatin was administered by intraperitoneal injection (IP) at a dose of 80 mg / kg and a volume of 10 mL / kg. Saline solution (0.9% NaCl) was used as a vehicle control for ODSH and was administered by the same route and in the same vo...
Embodiment 3
[0267] 6.3 Example 3: ODSH, a heparanoid that interacts with PF4, alleviates thrombocytopenia and neutropenia, induces thrombopoiesis and neutrophil production and reduces exposure to chemotherapy regimens with myelosuppressive side effects Patient's systemic symptoms
[0268] In an unblinded clinical trial, patients diagnosed with metastatic pancreatic cancer were treated with ODSH as gemcitabine and nab-paclitaxel ( Adjuvant therapy with albumin-bound paclitaxel).
[0269] Inclusion criteria Male and non-pregnant, non-lactating female patients aged 18 to 75 years with histologically confirmed metastatic adenocarcinoma participated in the trial. Other inclusion criteria were: presence of at least one metastatic tumor (measurable by conventional techniques or CT scan), serum CA 19-9 measurement greater than 2 times the upper limit of normal, no local targeted therapy within six months of participation in the trial Radiotherapy or chemotherapy for advanced disease, absolute ...
Embodiment 4
[0321] 6.4 Example 4: ODSH relieves thrombocytopenia associated with the treatment of acute myelogenous leukemia (AML)
[0322] The clinical trial was conducted to confirm the therapeutic advantages of ODSH administered as adjuvant induction and consolidation therapy and adjunctive subsequent treatment of allogeneic or autologous bone marrow transplantation in acute myeloid leukemia (AML). Subjects included in this experiment were those diagnosed with AML who were receiving induction and consolidation therapy. Subjects were randomly assigned to the control group (receiving induction and consolidation therapy only) or the treatment group (receiving adjuvant ODSH). ODSH was administered by continuous infusion (0.375 mg / kg / hr). Subjects in each group of the experiment were evaluated for platelet counts and the need for platelet transfusions. Other parameters assessed included measuring circulating levels of PF4 and granulocyte recovery.
[0323] Results were obtained demonstra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com