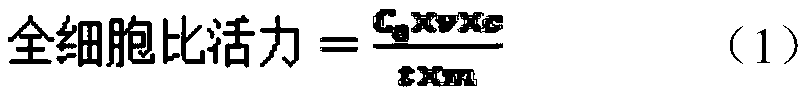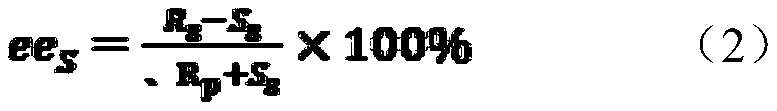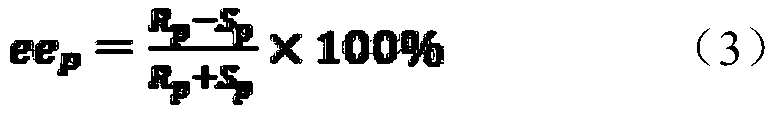A Mutant of Phaseolin Epoxyhydrolase with Improved Stereoselectivity
A technology of hydrolase and mutant, applied in the field of enzyme engineering, can solve the problem of not being able to produce high enantiopurity epoxides and vicinal diols, etc., and achieve the effect of improving enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Mutant plasmid construction
[0032]The E.coli BL21(DE3) / pET-28a-pveh1 (Ye Huihua, Hu Die, Li Chuang, etc.) preserved from the laboratory using the PurePlasmidMini Kit (purchased from Kangwei Reagent Co., Ltd.). Heterologous expression and enantio-normalized catalytic properties of oxide hydrolase[J]. China Biotechnology, 2016, 36(10): 21-27. 169: 41-54. template,
[0033] The primers used were P184L-F (5'-3'GAGACCAGGACCACTAATACTCCCCAA); P184L-R (5'-3'TTGGGGAGTATTAGTGGTCC TGGTCTC).
[0034] use HS PCR enzyme (purchased from TaKaRa Company) adopts the method of whole plasmid PCR. Using the PvEH1 plasmid as a template, PCR conditions: denaturation at 98°C for 10 s; annealing at 55°C for 5 s; extension at 72°C for 3.5 min, 30 cycles, and extension at 72°C for 10 min. The PCR product was transformed into E.coli BL21 (DE3) competent cells by digesting the template plasmid with Dpn I enzyme, and the transformation was performed according to the instruction man...
Embodiment 2
[0035] Embodiment 2: the acquisition of mutant enzyme
[0036] The obtained mutant enzyme expression vector was inoculated (the inoculum size was 1%) in 2 mL of LB medium containing 1% kanamycin, at 37 ° C, 220 r min -1 Cultivate overnight; take 2mL culture medium and transfer to 100mL LB medium containing 1% kanamycin, cultivate to OD 600 When it is 0.6~0.8, add 100μL of IPTG (500mmol·L -1 ) to a final concentration of 0.5mmol L -1 After induction at 20°C for 10 h, the recombinant bacterial cells were collected by centrifugation. It was determined that the enzyme activity of the epoxide hydrolase of the bacteria to rac-pCSO was 10.0 U / g of the bacteria cells.
Embodiment 3
[0037] Example 3: Enantioselectivity comparison of enzymes before and after mutation to rac-pCSO
[0038] The invention compares the stereoselectivity of the enzyme before and after the mutation, and the stereoselectivity includes two properties of enantioselectivity and enantionormalization. Wherein the wild enzyme refers to PvEH1 derived from Phaseolus vulgaris, GenBank Accession No. XM007146940.
[0039] Use 1ml of sodium phosphate buffer solution (pH=7.0, 100mmol·L) for every 20mg of wet bacteria -1 ) suspension, take 1mL and add it to a 2mLEP tube, add 50μL of 200mmol·L -1 rac-pCSO (solvent is methanol), the final substrate concentration is 10mmol L -1 . Placed at 25°C, 220r·min -1 Shaking table reaction, 50 μL samples were extracted three times with ethyl acetate (1 mL in total) at 30 min, 1 h, 2 h and 24 h respectively, dried with anhydrous magnesium sulfate and analyzed by high performance liquid chromatography. When the conversion rate c was about 50%, the E valu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com