Combined therapeutic use of antibodies and endoglycosidases
A serum antibody, therapeutic antibody technology, applied in the direction of antibody medical components, antibodies, immunoglobulins, etc., can solve problems such as the availability of unbound receptors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
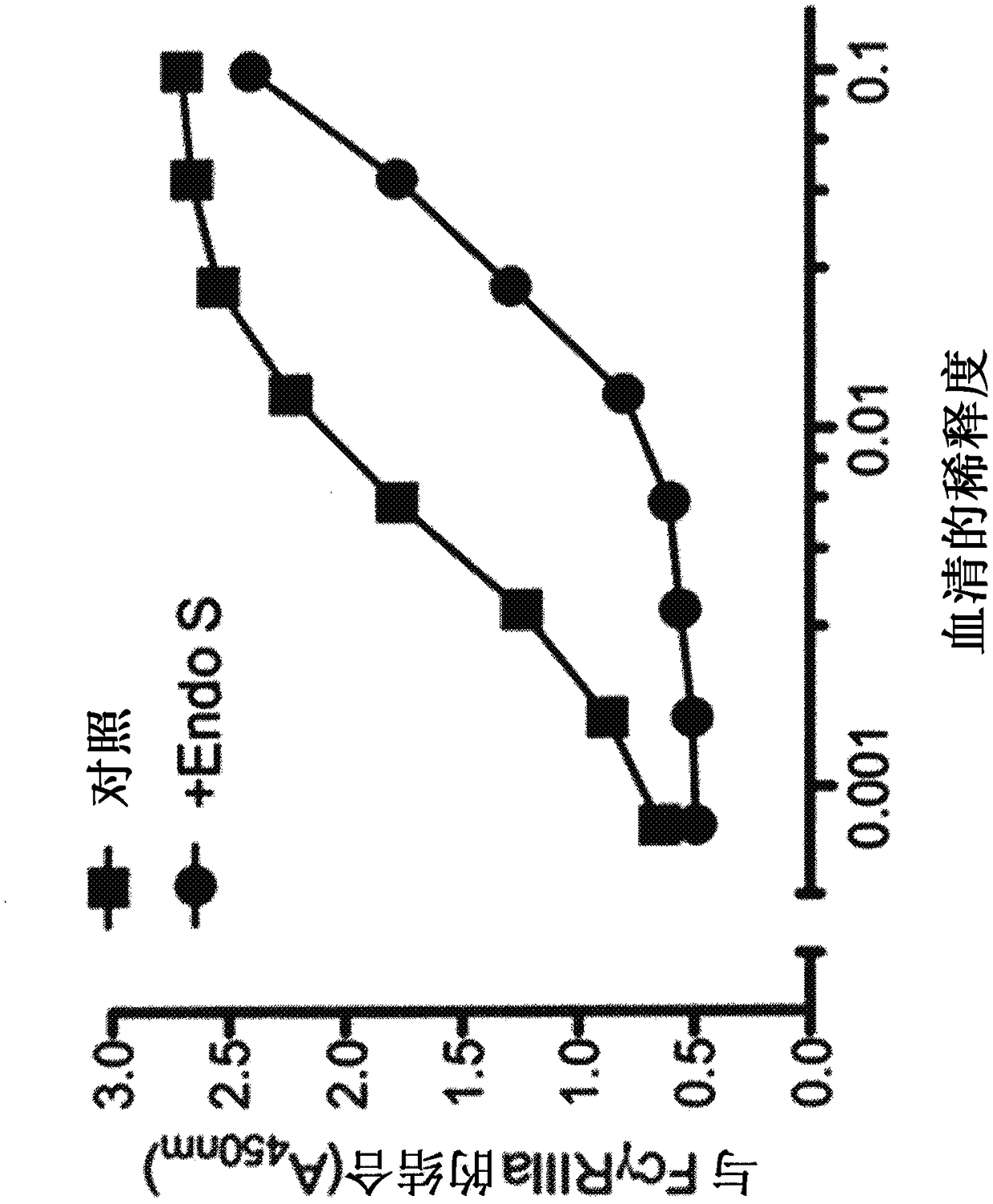
[0200] Example 1: Decreased binding of serum IgG to FcγRIIIa increases binding of resistant therapeutic antibodies
[0201] To demonstrate that EndoS can be used to reduce the binding of serum IgG to FcγRIIIa, the following experiments were performed. High binding microtiter plates (3690, Corning, NY, U.S.A.) were coated overnight at 4°C with 2.5 μg / mL of FcγRIIIa (158Val variant; R&D systems, Minneapolis, U.S.A.) in PBS. Coated plates were washed with PBS containing 0.05% Tween 20 (Sigma-aldrich, U.S.A.) and blocked with 3% BSA in PBS for 2 hours at room temperature. Then add human serum (H4522, Sigma-Aldrich, U.S.A.) or carry Man 9 GlcNAc 2 or Man 5 GlcNAc 2 serial dilutions of recombinant human IgG1 glycoforms (initial concentration 0.1 mg / mL in PBS) and allowed to bind for 2 hours at room temperature. Plates were washed five times with PBS containing 0.05% Tween and binding was detected using a Fab fragment specific for murine IgG Fab conjugated to HRP (ab98659, Abcam...
Embodiment 2
[0221] Example 2: Mouse Model
[0222] Cells from the cell line SKBR3 (a cell line highly expressing HER2) were introduced subcutaneously into mice. EndoS and a therapeutic antibody against HER2 (such as Herceptin) resistant to EndoS are introduced intravenously at a dose of 30 mg / Kg per week for 4 weeks. Control mice did not receive (i) Endo S or (ii) therapeutic antibody. The size of the resulting tumors was measured with calipers.
Embodiment 3
[0223] Example 3: Delayed Administration of Therapeutic Antibodies
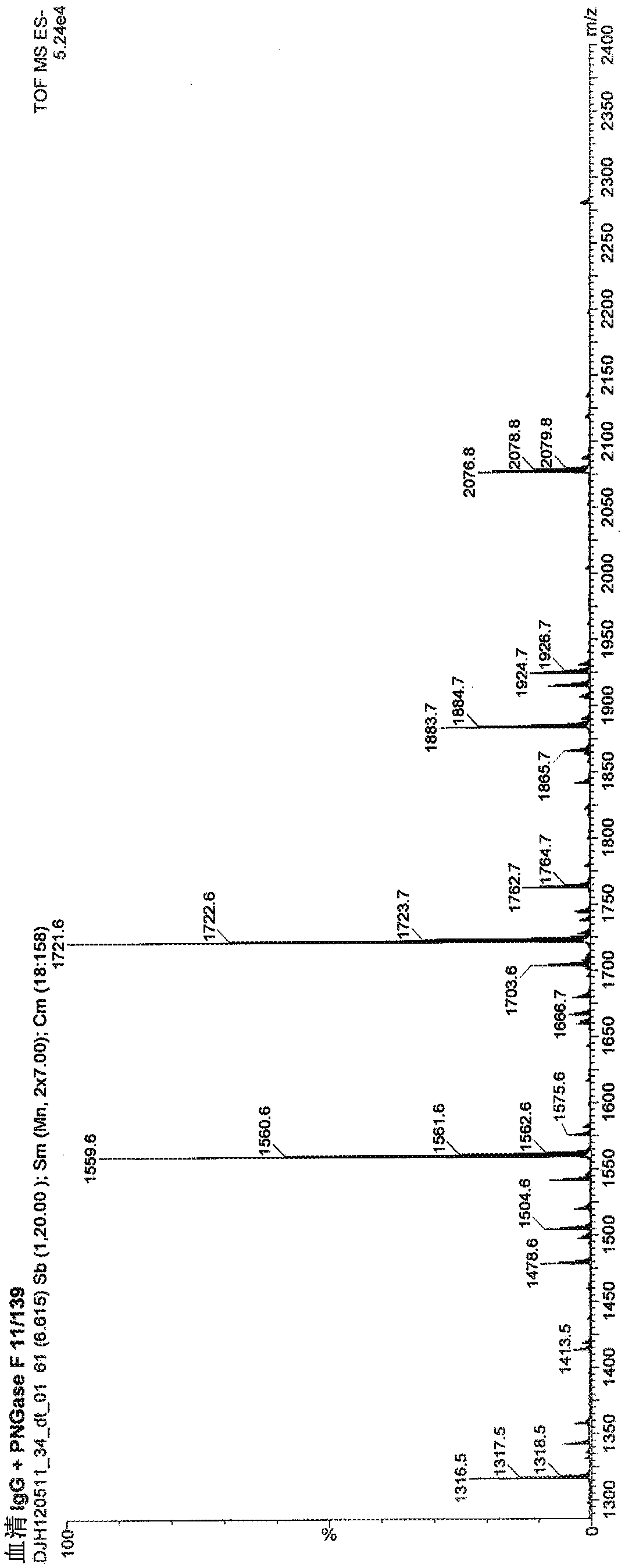
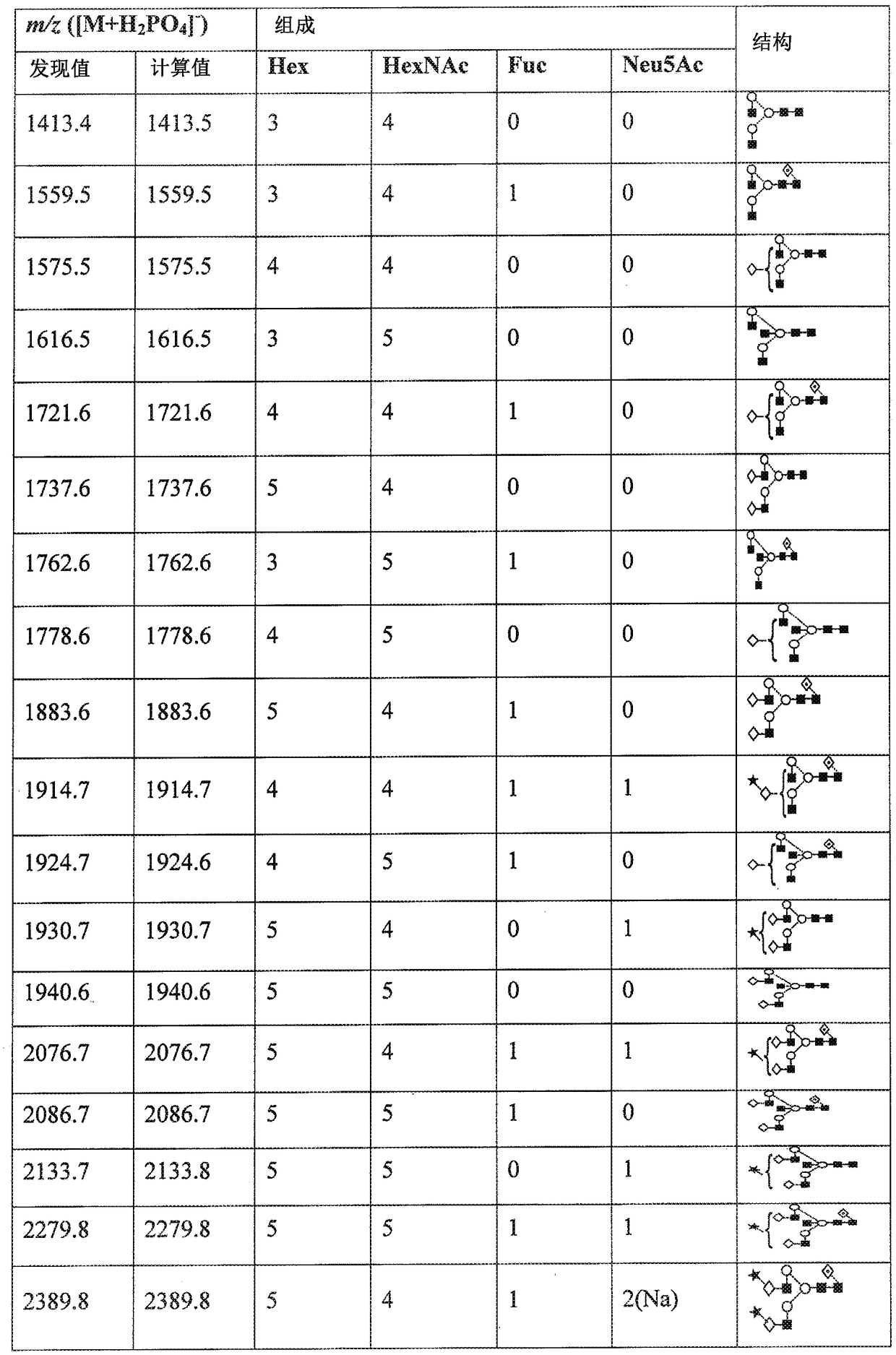
[0224] Breast cancer subjects were treated intravenously with 15 mg EndoS in saline solution. Monitor patient IgG glycosylation levels (deglycosylation of IgG reduces IgG mass) by purifying patient IgG samples and estimating IgG mass by SDS-PAGE.
[0225] After a 2-day delay, subjects were treated intravenously with 2 mg of therapeutic antibody / kg body weight of trastuzumab.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com