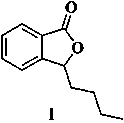
Preparation method of butylphthalide and pharmaceutical intermediate thereof
A technology of intermediates and drugs, which is applied in the field of medicinal chemistry, can solve problems such as unsuitable for large-scale industrial production, unavailable raw materials, and high toxicity of cyanide ketones, so as to facilitate large-scale industrial production, avoid the use of Grignard reagents, The effect of reducing production energy consumption and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 2-(methoxycarbonyl) benzoic acid
[0041] Add 500g of phthalic anhydride to a 3L reaction flask, add 2L of methanol to the system, raise the temperature of the system to 60-80°C until the phthalic anhydride disappears, concentrate under reduced pressure to remove methanol, and obtain a wet product at 50°C Vacuum drying for 4-6 hours gave 588 g of 2-(methoxycarbonyl)benzoic acid, with a yield of 96.7%.
Embodiment 2
[0042] Example 2 Preparation of 2-(ethoxycarbonyl)benzoic acid
[0043] Add 500g of phthalic anhydride to a 3L reaction flask, add 2L of ethanol to the system, raise the temperature of the system to 60-80°C until the phthalic anhydride disappears, concentrate under reduced pressure to remove ethanol, and obtain a wet product at 50°C Vacuum drying for 4-6 hours gave 596 g of 2-(ethoxycarbonyl)benzoic acid, with a yield of 91.0%.
Embodiment 3
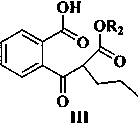
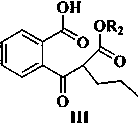
[0044] Example 3 2-[(2-Methoxycarbonyl)pentanoyl]benzoic acid
[0045] Add 428g of sodium hydride into a 5L reaction flask, add 3.1L of N,N-dimethylformamide, 311g of methyl valerate and 580g of 2-(methoxycarbonyl)benzoic acid to the system at 0-10°C, The temperature of the system rises to 90-100°C, react at this temperature for 4-6 hours, stop heating, lower the system to room temperature, add 4L of purified water and stir until the system is dissolved, adjust the pH of the system to 2-3 with concentrated hydrochloric acid, ethyl acetate After ester extraction, the organic phase was separated, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated in vacuo to obtain 646 g of 2-[(2-methoxycarbonyl)pentanoyl]benzoic acid, with a yield of 91.2%. Purity 95.6% (HPLC method);
[0046] 1 HNMR (400MHz, CDCl 3 )7.84(t, 1H), 7.72(d, 1H), 7.63−7.52(m, 2H), 3.78(s, 3H, OCH 3 ), 2.93 (dd, 1H), 2.35–1.73 (m, 2H), 1.34–1.25 (m, 4H), 0.90 (t, 3H). LC-MS for C...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap