A kind of diphenol monomer containing asymmetric fluorophore structure and its preparation method and application
A fluorophore, asymmetric technology, applied in the field of electrochromic and electronically controlled fluorescence, can solve the problems of unstable electrical activity, slow, low fluorescence contrast development of electronically controlled fluorescent materials, and reduce the degree of aggregation quenching and accumulation. effect of enhanced performance of electrochromic, electrochromic and electronically controlled fluorescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The invention provides a preparation method of the diphenol monomer containing an asymmetric fluorophore structure, comprising the following steps:
[0042] 4-fluoronitrobenzene, R 2 -NH 2 , triethylamine and dimethyl sulfoxide are mixed, and a nucleophilic substitution reaction occurs to obtain a compound having a structure shown in formula II;
[0043] The compound having the structure shown in formula II, copper powder, potassium carbonate, 18-crown-6, R 1 -X is mixed with o-dichlorobenzene, and Ullmann reaction I occurs to obtain a compound with the structure shown in formula III; the R 1 X in -X is Cl, Br or I;
[0044] mixing the compound having the structure shown in formula III, Pd / C, hydrazine hydrate and dioxane, and performing a reduction reaction to obtain the compound having the structure shown in formula IV;
[0045] Mix the compound with the structure shown in formula IV, p-iodoanisole, copper powder, 18-crown-6, potassium carbonate and o-dichlorobenz...
Embodiment 1
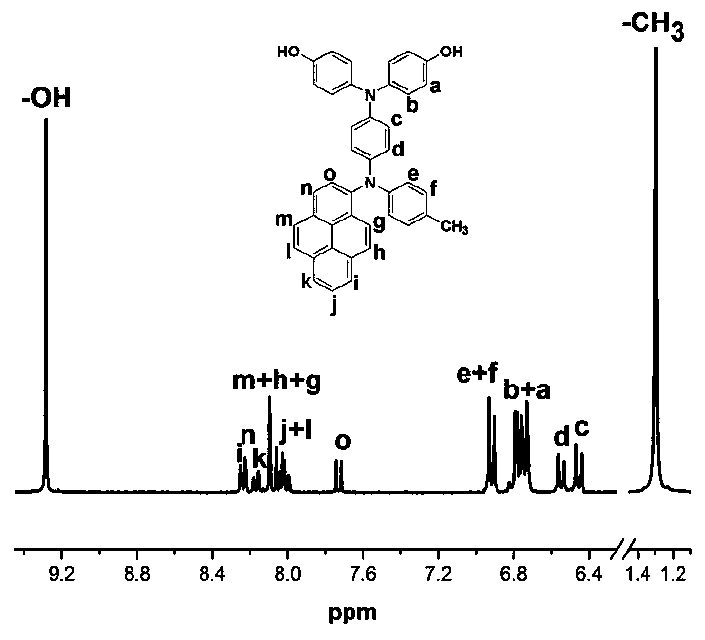
[0142] The preparation of N,N-bis(4-hydroxyphenyl)-N'-4-methylphenyl-N'-1-pyrenyl-1,4-phenylenediamine, its structural formula is as follows:
[0143]
[0144] Under nitrogen, add 16.9g (120mmol) of 4-fluoronitrobenzene, 16.7g (156mmol) of p-methylaniline, 15.8g (156mmol) of triethylamine in a 250mL three-necked flask equipped with mechanical stirring , adding 130 mL of dimethyl sulfoxide as a solvent, and reacting at 85° C. for 36 h. Discharge in ice-water mixture at room temperature, filter with suction, and recrystallize the filter cake with ethanol and N,N-dimethylformamide to obtain 21.9 g of 4-nitro-4'-methyldiphenylamine with a yield of 80% ;
[0145] Under nitrogen, add 10.0 g (35.6 mmol) of 1-bromopyrene, 9.0 g (37.4 mmol) of 4-nitro-4'- Copper powder of methyl diphenylamine, 9.0g (142.2mmol), 19.6g (142.2mmol) of potassium carbonate, 4.7g (17.8mmol) of 18-crown-6 are raw materials, add 60mL of o-dichlorobenzene as solvent, 160 Reaction at ℃ for 18h; Suction fil...
Embodiment 2
[0151] The preparation of N,N-bis(4-hydroxyphenyl)-N'-2-anthracenyl-N'-9-anthracenyl-1,4-phenylenediamine, its structural formula is as follows:
[0152]
[0153] Under nitrogen, add 7.1g (50.0mmol) of 4-fluoronitrobenzene, 14.5g (75.0mmol) of 2-aminoanthracene, 7.6g (75.0mmol) of Triethylamine was then added to 58.9 mL of dimethyl sulfoxide, and reacted at 95°C for 42h. Discharge in ice-water mixture at room temperature, filter with suction, and recrystallize the filter cake with ethanol and N,N-dimethylformamide to obtain 12.9 g of N-4-nitrophenyl-2-anthracenamine, yield 82 %;
[0154] Under nitrogen, add 7.8g (30.3mmol) of 9-bromoanthracene, 12.0g (36.3mmol) of N-4-nitrophenyl -2-Anthracene amine, 9.6g (151.5mmol) copper powder, 20.9g (151.5mmol) potassium carbonate, 5.6g (21.2mmol) 18-crown-6, then add 69.4ml of o-dichlorobenzene, 155°C Reaction for 14h; Suction filtration while hot, distilled o-dichlorobenzene under reduced pressure, the obtained crude product was r...
PUM
| Property | Measurement | Unit |
|---|---|---|
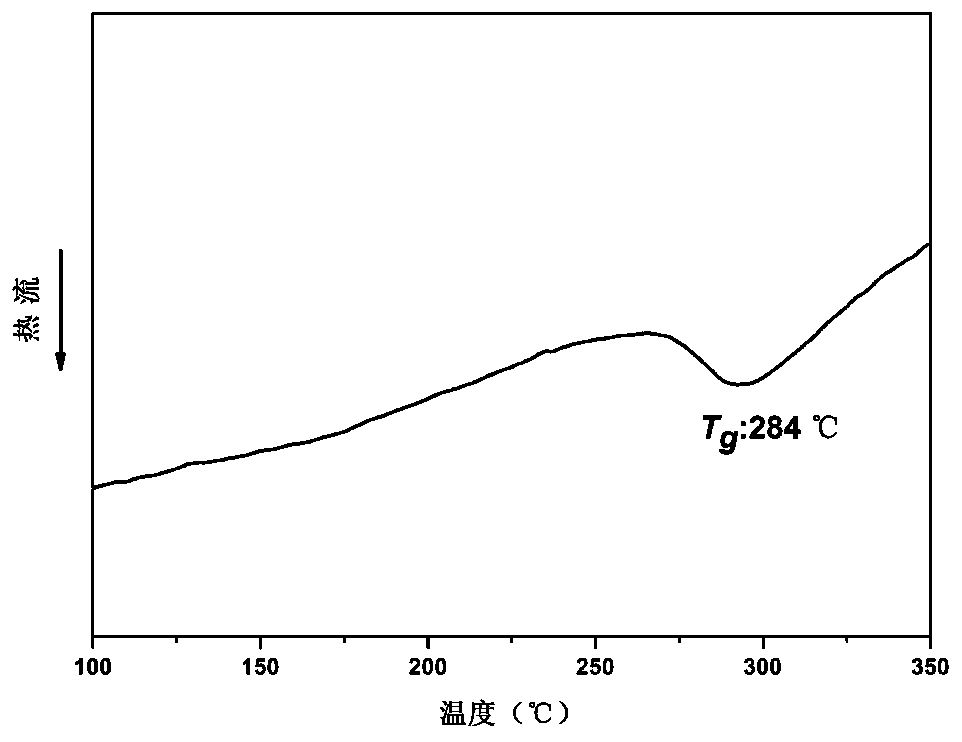
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com