Intra-articular administration of polymetaphopsphates for the treatment of crystal arthropaties
A technology of polymetaphosphate and joint disease, applied in the field of polymetaphosphate, can solve the problems of difficult preparation of protein source and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
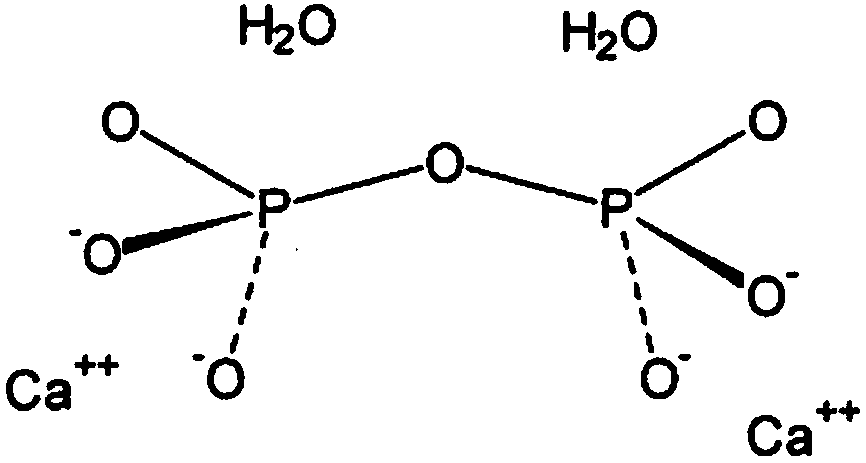
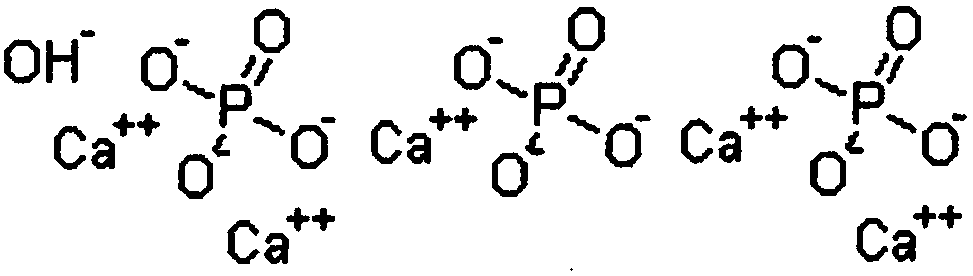
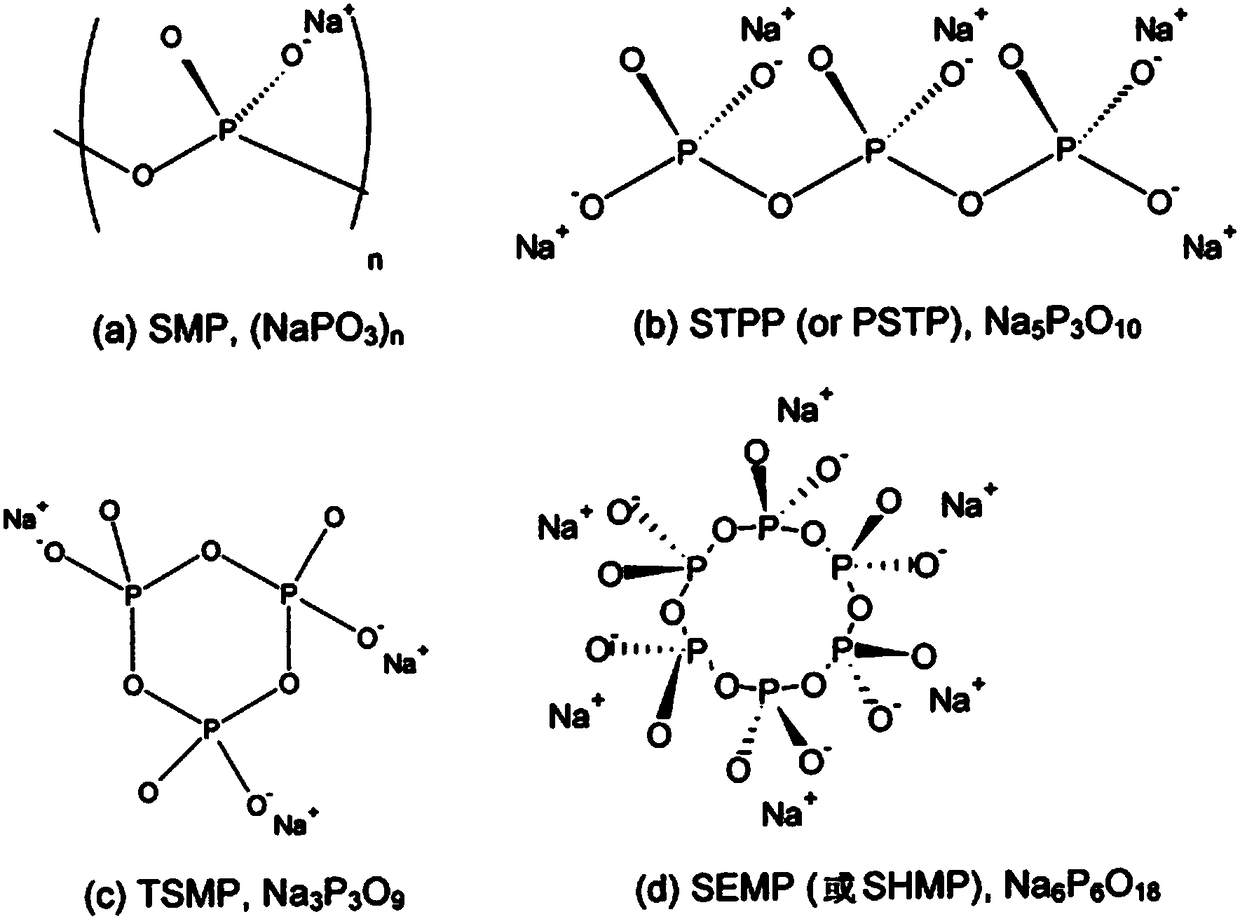
[0027] Accordingly, the present invention provides, inter alia, a formulation for the treatment of arthropathy caused by microcrystalline deposition comprising a diluent aqueous sterile solution and formula M in the form of a sterile lyophilized powder n+2 P n o 3n The linear polymetaphosphate or formula (MPO 3 ) n cyclic polymetaphosphate, wherein M represents an alkali metal, wherein the volume of diluent solution is 100ml to 1500ml, and the weight / volume ratio of lyophilized polymetaphosphate powder to diluent solution is 1:1000 to 10:1000 , and wherein said treatment is based on reconstitution of polymetaphosphate powder in said diluent solution by
[0028] a) by stepwise lavage, i.e. by sequentially applying prescribed portions of said reconstitution solution to lavage the joint; or
[0029] b) Continuous lavage with the reconstituted solution.
[0030] The polymetaphosphate according to the invention is an alkali metal metaphosphate, preferably the sodium salt; more...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com