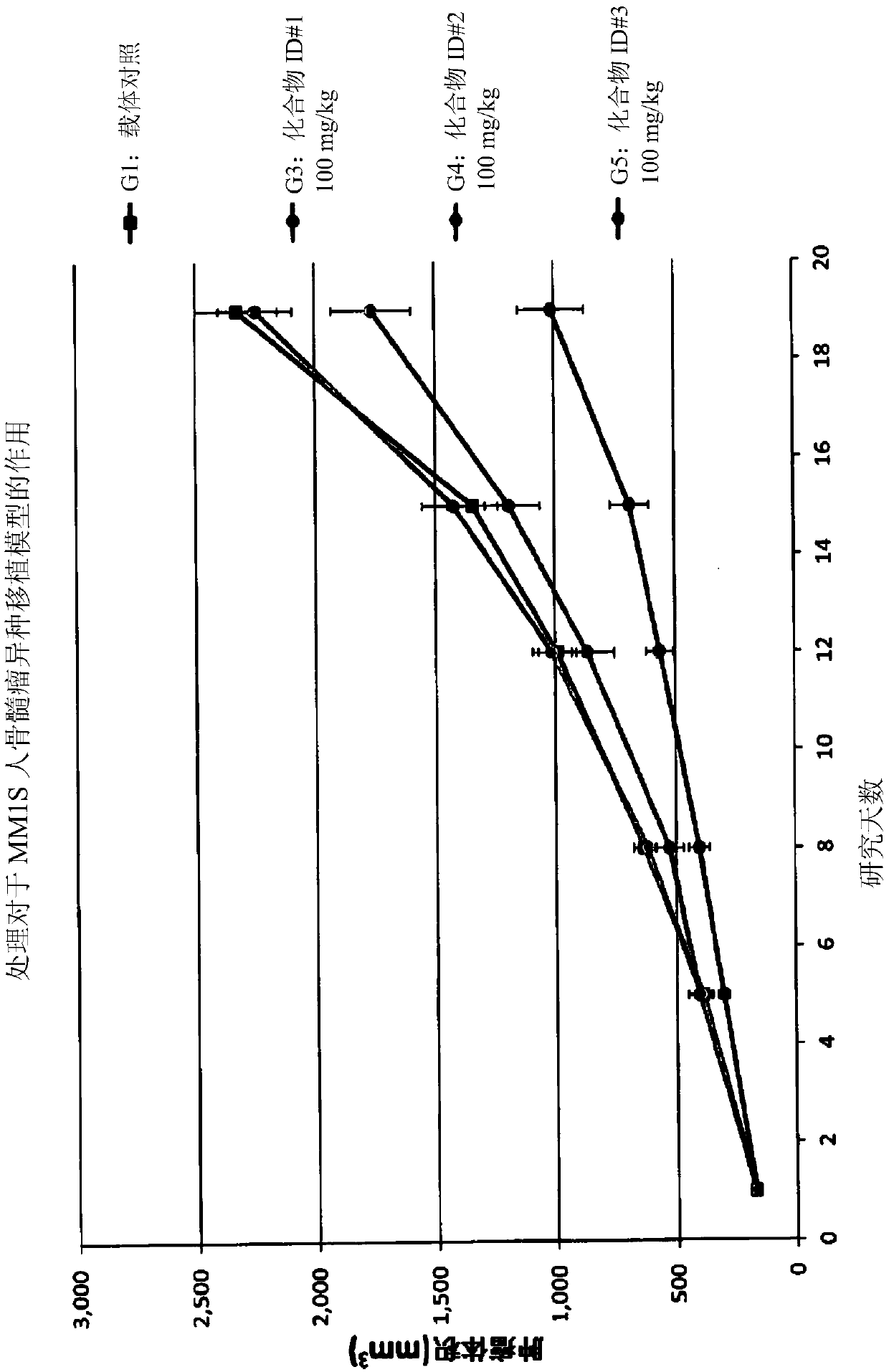
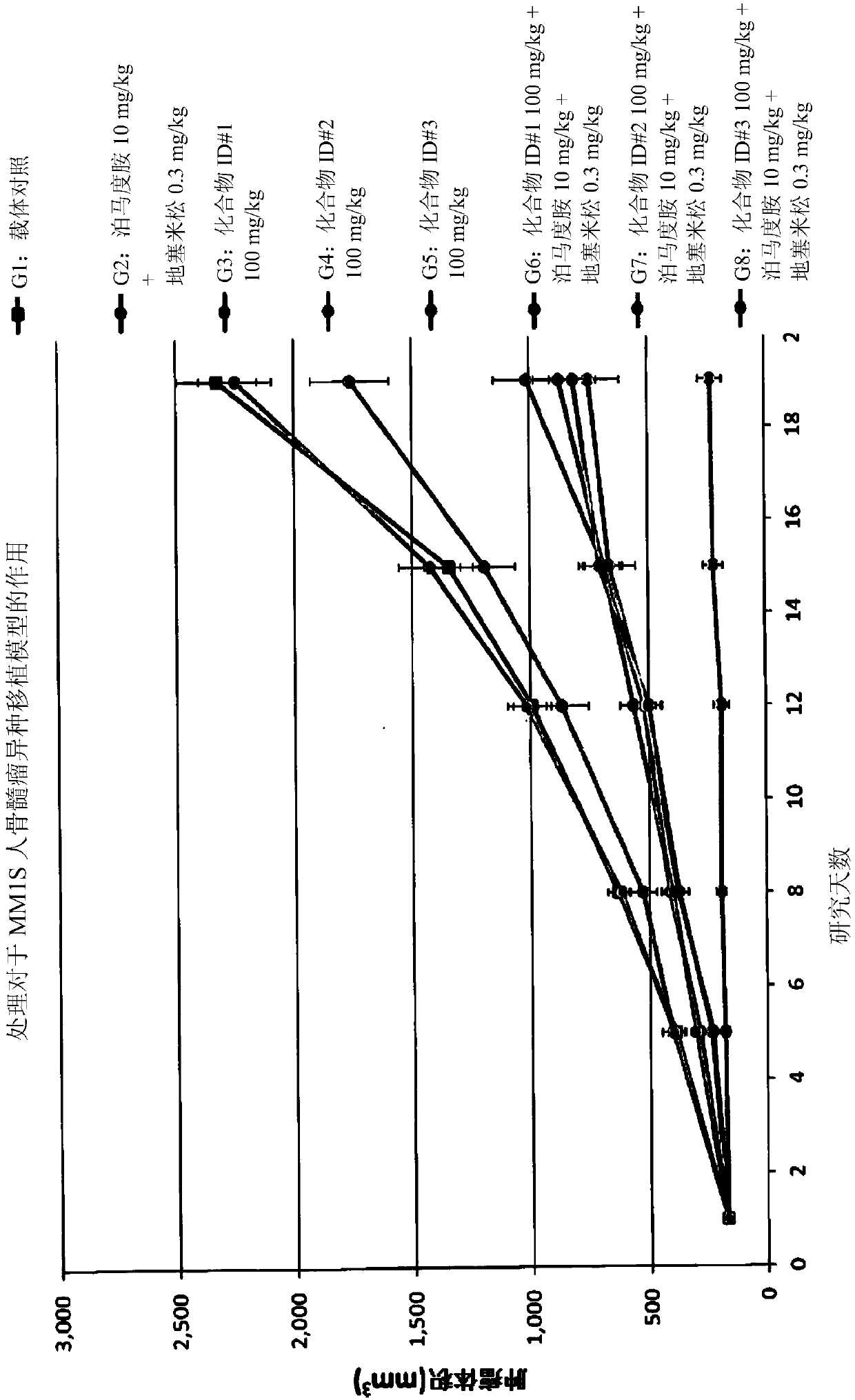
Aminobenzimidazole derivatives
A benzo, hydroxybenzamide technology, applied in the directions of drug combination, organic chemistry, organic active ingredients, etc., can solve the problems of difficult to treat cancer, undesired side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
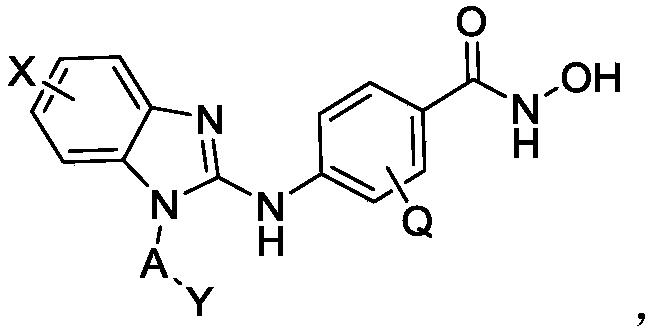
[0088] Example 1. Exemplary Compounds of Formula (I) or Formula (II)
[0089] 4-((1-(cyclohexylmethyl)-1H-benzo[d]imidazol-2-yl)amino)-N-hydroxybenzamide. ID#1
[0090]
[0091] 4-((1-cyclohexyl-1H-benzo[d]imidazol-2-yl)amino)-N-hydroxybenzamide. ID # 2
[0092]
[0093] 4-((1-cycloheptyl-1H-benzo[d]imidazol-2-yl)amino)-N-hydroxybenzamide. ID#3
[0094]
[0095] 4-((1-((1-fluorocyclohexyl)methyl)-1H-benzo[d]imidazol-2-yl)amino)-N-hydroxybenzamide. ID#4
[0096]
[0097] 4-((1-(cyclopentylmethyl)-1H-benzo[d]imidazol-2-yl)amino)-N-hydroxybenzamide. ID#5
[0098]
[0099] 4-((1-(2-cyclopentylethyl)-1H-benzo[d]imidazol-2-yl)amino)-N-hydroxybenzamide. ID#6
[0100]
[0101] 4-((1-((4,4-difluorocyclohexyl)methyl)-1H-benzo[d]imidazol-2-yl)amino)-N-hydroxybenzamide ID#7
[0102]
[0103] 4-((1-((4,4-difluorocyclohexyl)methyl)-5-fluoro-1H-benzo[d]imidazol-2-yl)amino)-N-hydroxybenzamide. ID#8
[0104]
[0105] N-Hydroxy-4-((1-((tetrahydro-2H-pyran-4-y...
Embodiment 2
[0109] Example 2. Cell viability assay using MM1.S cells
[0110] Cell viability was used to assess cytotoxicity and the effect of compounds on cell proliferation in the presence of various concentrations of the above compounds at different time points. IC of disclosed compounds in MM1.S cell line 50 (or percent activity) data are summarized in Table 1.
[0111] Cell viability assay - by Promega (Madison, WI) The cell viability assay measures cell viability. The luminescent cell viability assay is a homogenous method used to determine the number of surviving cells in culture based on the quantification of the ATP present, which indicates the presence of metabolically active cells. After processing, the Add to treated wells and incubate at 37°C. Luminescence was measured using a Molecular Devices Spectramax microplate reader.
[0112] Single Reagent Studies - Cells were grown to 70% confluency, trypsinized, counted, and plated in 96-well flat-bottom plates at a final...
Embodiment 3
[0122] Example 3. Cell Viability Assay Using Various Cell Lines
[0123] Compound ID #1 (compound of formula (I)) and compound ID #3 (compound of formula (II)) were tested for inhibition of cancer cell proliferation. Cell viability was used to assess cytotoxicity and the effect of compounds on cell proliferation in the presence of various concentrations of Compound ID#1 and Compound ID#3 at different time points. The 50% inhibitory concentration (IC) of the compound 50 ) data are summarized in Table 2. The data clearly show the surprising and unexpected increased anticancer activity associated with Compound ID#1 compared to Compound ID#3.
[0124] Cell viability assay - by Promega (Madison, Wis.) The cell viability assay measures cell viability. The luminescent cell viability assay is a homogenous method used to determine the number of surviving cells in culture based on the quantification of the ATP present, which indicates the presence of metabolically active cells. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com