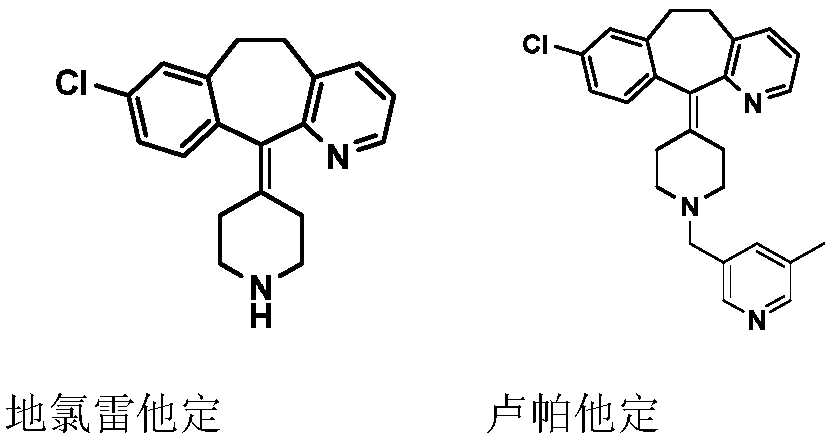
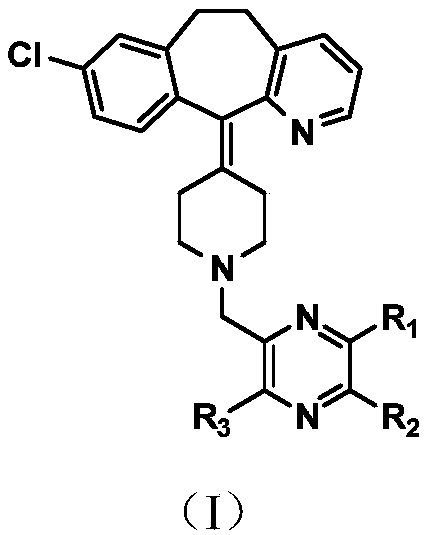
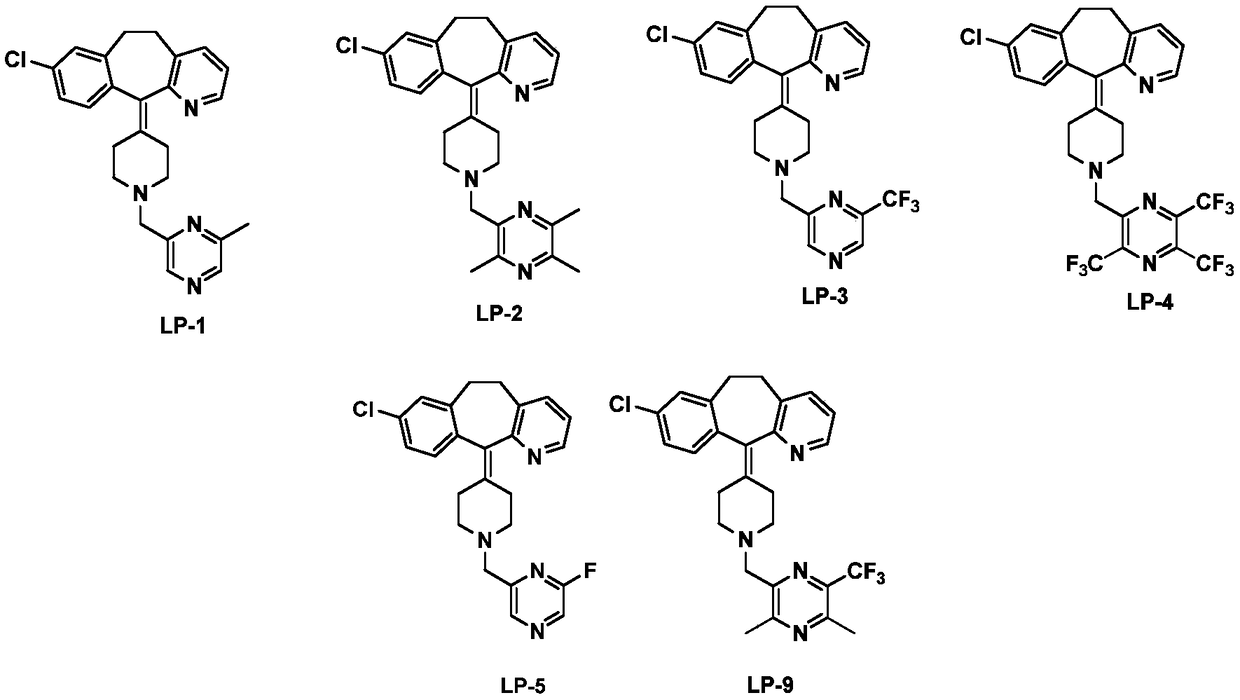
Compound with dual antagonistic activity of histamine receptor and application
A compound and drug technology, applied in the field of medicinal chemistry, can solve the problems of weak anti-inflammatory effect and decreased body resistance, and achieve the effects of enhanced anti-inflammatory activity, significant anti-inflammatory effect, and good medicinal prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 8-Chloro-6,11-dihydro-11-(1-(6-methylpyrazin-2-yl)methyl)piperidin-4-ylidene)-5H-benzo[5,6]ring Synthesis of Hepta[1,2-b]pyridine (LP-1)
[0045] Synthesis of step 1 intermediate 2-(chloromethyl)-6-methylpyrazine
[0046]
[0047] SM (500mg, 4.62mmol), NCS (617mg, 4.53mmol) and carbon tetrachloride (13mL) were sequentially added to a 50mL single-necked bottle, and heated to reflux under nitrogen protection. BPO (22mg, 0.077mmol) was added to the above reaction solution, and the reaction was refluxed for 3h. NCS (62mg, 0.45mmol) was added to the above reaction solution, refluxed for 2h and then transferred to room temperature for overnight reaction. After the reaction was monitored by TLC (PE:EA=4:1), water (10 mL) was added to the reaction solution, extracted with dichloromethane (10 mL×3), and the organic layers were combined. Wash with saturated sodium chloride solution (5 mL×3), and collect the organic phase. Dry over anhydrous magnesium sulfate, filter with s...
Embodiment 2
[0052] 8-Chloro-6,11-dihydro-11-(1-((3,5,6-trimethylpyrazin-2-yl)methyl)piperidin-4-ylidene)-5H-benzo Synthesis of [5,6]cyclohepta[1,2-b]pyridine (LP-2)
[0053] With reference to the method of Example 1, 2,6 dimethylpyrazine was replaced by 2,3,5,6-tetramethylpyrazine, and the compound 8-chloro-6,11-dihydro-11-(1 -((3,5,6-trimethylpyrazin-2-yl)methyl)piperidin-4-ylidene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine (LP-2) (103 mg, 51.5% yield), pale yellow solid. 1 H NMR 400MHz (CDCl 3 )δ9.01(s,1H),8.82(s,1H),8.41(dd,J=1.6Hz,J=4.8Hz,1H),7.45(dd,J=1.6Hz,J=7.6Hz,1H) ,7.13(d,J=7.6Hz,2H),3.80(s,2H),3.33-3.42(m,2H),2.76-2.88(m,4H),2.53-2.60(m,10H),2.79-2.51 (m,5H)
Embodiment 3
[0055] 8-Chloro-6,11-dihydro-11-(1-(6-trifluoromethylpyrazin-2-yl)methyl)piperidin-4-ylidene)-5H-benzo[5,6 Synthesis of ]cyclohepta[1,2-b]pyridine (LP-3)
[0056] With reference to the method of Example 1, 2,6 dimethylpyrazine was replaced by 6-trifluoromethylpyrazine, and the compound 8-chloro-6,11-dihydro-11-(1-(6-tri Fluoromethylpyrazin-2-yl)methyl)piperidin-4-ylidene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine (LP-3) (36mg, 40.9 % yield), white solid. 1 H NMR 400MHz (CDCl 3 )δ9.02(s,1H),8.83(s,1H),8.40(dd,J=1.6Hz,J=4.8Hz,1H),7.45(dd,J=1.6Hz,J=7.6Hz,1H) ,7.13-7.16(m,3H),7.07-7.10(m,1H),3.80(s,2H),3.33-3.42(m,2H),2.76-2.88(m,4H),2.53-2.60(m, 1H),2.79-2.51(m,5H)
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap