Recombinant antigen composition for detecting herpes simplex virus type-II IgG antibody, and kit thereof
A herpes simplex virus and recombinant antigen technology, applied in the field of immunological diagnosis, can solve the problems of low sensitivity and false positives of HSV antibodies, and achieve the effects of improving sensitivity and specificity, improving sensitivity and reducing the probability of false positives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] A recombinant antigen composition for detecting herpes simplex virus type II IgG antibody, the recombinant antigen composition includes the recombinant antigen of the antigenic epitope of glycoprotein gG and the recombinant antigen of the antigenic epitope of glycoprotein gD.
[0019] Wherein the amino acid sequence of the antigenic epitope of glycoprotein gG is shown in SEQ ID NO:1, and the nucleotide sequence encoding the amino acid sequence is shown in SEQ ID NO:2.
[0020] Wherein the amino acid sequence of the antigenic epitope of glycoprotein gD is shown in SEQ ID NO:3, and the nucleotide sequence encoding the amino acid sequence is shown in SEQ ID NO:4.
[0021] The preparation method of the recombinant antigen composition comprises the following steps:
[0022] (1) Screening of diagnostic epitopes of herpes simplex virus type II IgG antibodies
[0023] Using bioinformatics analysis tools, analyze the full-length amino acid sequences of the herpes simplex virus ...
Embodiment 2
[0034] A kit for detecting herpes simplex virus type II IgG antibody, the kit includes: a recombinant antigen composition-coated microtiter plate, negative control serum 100ul, positive control serum 100ul, bovine serum albumin 10.0mg, horseradish over Oxidase-labeled mouse anti-human IgG10ul, sample diluent 90ml (0.01M pH7.4 phosphate buffer, 10% fetal bovine serum), 100× concentrated washing buffer 20ml (1M pH7.4 phosphate buffer , 5% Tween20), chromogenic solution A50ml (citric acid 0.2M, citric acid 16.65mM, 0.018% hydrogen peroxide), chromogenic solution B50ml (0.54mM sodium edetate, 5mM citric acid, glycerol 10%, 0.96mM TMB), stop solution 50ml (2M H 2 SO 4 ).
[0035] Enzyme plate coating:
[0036] The protein gG and gD purified by the nickel column were mixed at a concentration of 0.1 ug / ml, and then coated with a 96-well microtiter plate (Nunc company) at a volume ratio of 1:1, 100 ul per well, overnight at 4 ° C, washed with washing solution ( PBS-0.05% Tween20) ...
Embodiment 3
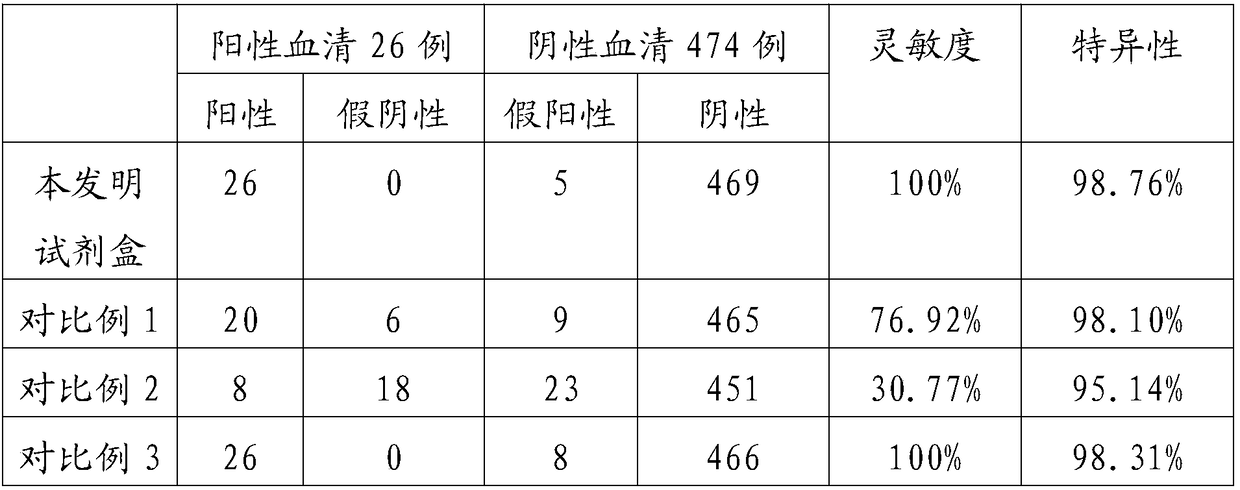
[0046] Embodiment 3 detects the performance detection of the kit of herpes simplex virus type II IgG antibody
[0047] (1) Test on the stability of the kit
[0048] The kit in Example 2 was refrigerated, and the storage stability test of the kit was carried out. The negative serum and positive serum were used to test the OD value of the same batch number at different storage times, and the results are shown in Table 1 below.
[0049] Table 1
[0050]
[0051] The above results clearly show that the OD value of the kit prepared from the recombinant antigen composition is still very stable after being stored for 13 months. In addition, in the tests using negative serum and positive serum, it can be observed that the OD value tends to increase gradually.
[0052] (2) Inspection of the intra-assay and inter-assay precision of the kit
[0053] Inter-batch precision investigation
[0054] Three quality control samples with different OD values were selected as determ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap