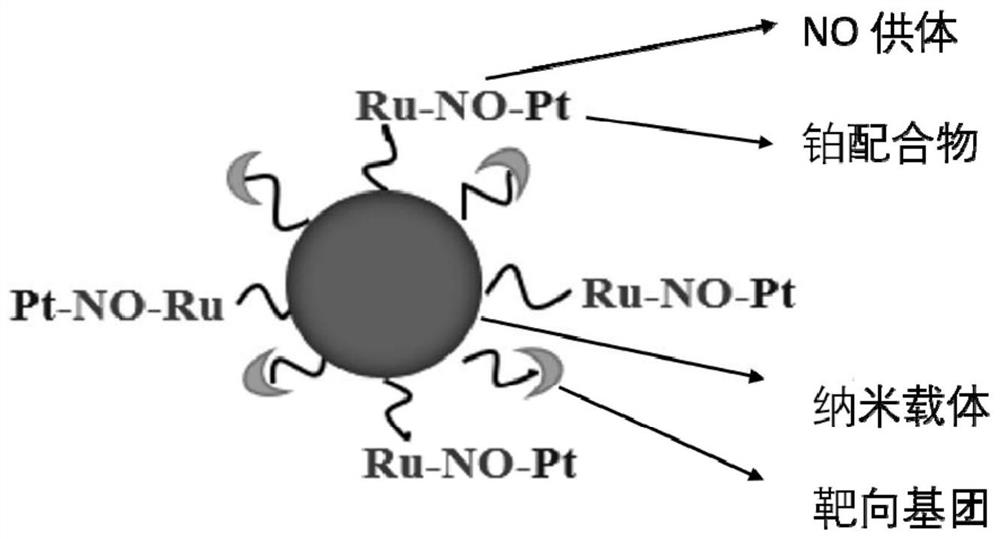
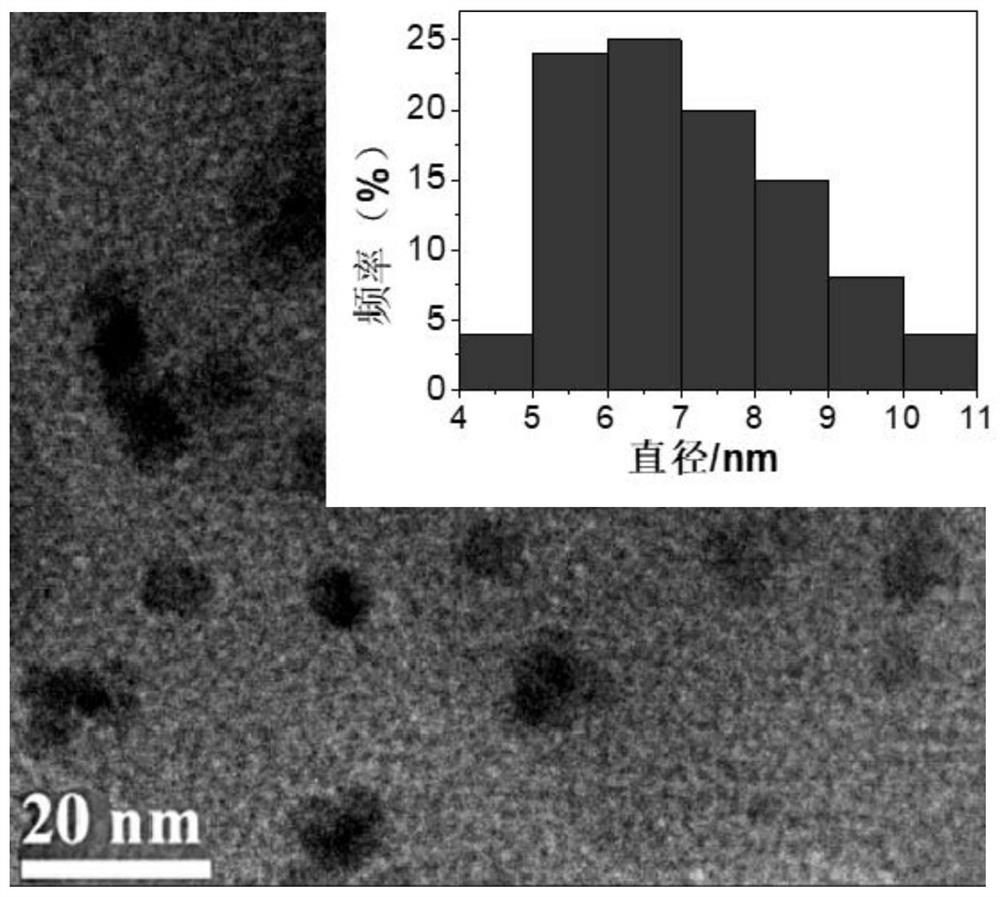
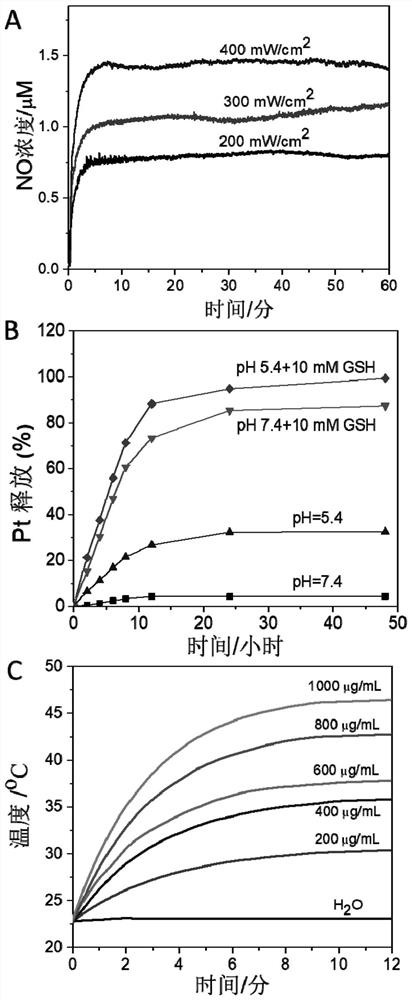
Nitric oxide and cisplatin targeted combined controllable delivery nano drug system and its preparation
A nano-carrier and drug technology, applied in the field of multifunctional bimetallic nano-composite drug system and its preparation, to achieve good biocompatibility, stability, and high-efficiency anti-cancer effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0141] The present invention also provides a preparation method of the pharmaceutical system of the present invention, which generally includes the following steps:
[0142] (1) Provide metal nitrosyl NO donors, platinum complexes, targeting groups and carrier nanoparticles;
[0143] (2) covalently loading the metal nitrosyl NO donor, the platinum complex, the targeting group and the carrier nanoparticles to form the bimetallic nanocomposite drug system .
[0144] In another preferred example, in step (1), the metal nitrosyl NO donor includes a metal nitrosyl compound (A)[(tpy')M 1 (R 1 )(NO)](PF 6 ) 3 , tpy' is 4'-formic acid-2,2':6',2"-terpyridine, M 1 For metal ruthenium (Ru), R 1 For 3-formic acid-o-phenylenediamine or its derivatives.
[0145] In another preferred example, in step (1), the platinum complex includes metal compound (B) cis-[M 2 (NH 3 ) 2 Cl 2 (OH) 2 ], M 2 For the metal platinum (Pt).
[0146] In another preferred example, the carrier nanopart...
Embodiment 1
[0159] Metal Ru nitrosyl compounds [(tpy COOH )Ru(MDAB)(NO)](PF 6 ) 3 Synthesis
[0160] (1)[(tpy COOH )Ru(MDAB)(Cl)](PF 6 )Synthesis
[0161] (tpy COOH is 4'-formic acid-2,2':6',2"-terpyridine; MDAB is methyl 3,4-diaminobenzoate):
[0162] Add Ru(tpy COOH ) Cl 3 (150mg, 0.31mmol), MDAB, (55mg, 0.33mmol), LiCl (5mg, 2.0mmol) and Et 3 N 0.4mL, vacuumize and nitrogen three times respectively, add 40mL of EtOH / H 2 O(3:1, v / v), in N 2 Reflux in the atmosphere for 8 hours, suction filter while hot, and concentrate the dark red filtrate to several milliliters. After cooling to room temperature, add excess saturated NH 4 PF 6 solution, and the mixture was placed in a refrigerator at 5°C overnight. The reddish-brown precipitate was filtered out and washed with H 2 O and Et 2 O were washed three times, respectively, and dried in vacuo. 137 mg of the target product was obtained with a yield of 61%.
[0163] (2)[(tpy COOH )Ru(MDAB)(NO 2 )](PF 6 )Synthesis:
[0164] W...
Embodiment 2
[0169] Synthesis of Nanoparticle Composite Drug System {N-GQDs@Ru-NO-Pt@FA}
[0170] (1) N-GQDs@NH 2 (Surface Aminated N-GQDs Nanoparticles) Preparation
[0171] Weigh citric acid (40 mg), dissolve it in 40 mL of distilled water, add ammonia water (8 mL), and place the solution in a muffle furnace at 200° C. for 3 h. After cooling to room temperature, the pH was adjusted to 8 to obtain a light yellow solution, which was dialyzed in water for 4 h with a dialysis bag with a molecular weight cut-off of 1000, and the solution was rotary evaporated to obtain N-GQDs as a solid. Dissolve N-GQDs in 2.0 mL of distilled water, add EDC / NHS, activate for 1 h, add 1 mL of anhydrous ethylenediamine, and stir at room temperature for 24 h. The solution was dialyzed in water for 24 hours with a dialysis bag with a molecular weight cut-off of 1000, and the solution in the dialysis bag was freeze-dried to obtain the product N-GQDs@NH 2 .
[0172] (2) Preparation of nanoparticle composite dru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com