Vector-free delivery of gene editing proteins and compositions to cells and tissues
A technology of gene editing and composition, applied in vectors, nucleic acid vectors, gene therapy, etc., can solve the problems of difficult detection of previous events, expensive regulatory procedures, and problems, and achieve good gene editing efficiency, less off-target effects, and survival good vitality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0207] Example 1: The data described herein were generated using the following materials and methods.
[0208] Cells and Cell Culture
[0209] A549 cells (A549 European Cell Culture Collection (ECACC) cells were purchased through Sigma-Aldrich Company (category number 86012804) (St.Louis, MO, USA) and Beas-2B (Sigma-Aldrich Company, catalog number 95102433), and Routine culture in DMEM (Gibco) supplemented with 5% fetal calf serum and 2mM L-glutamine (Gibco). Jurkat cells (European Collection of Cell Cultures; ECACC) were incubated at 37°C, 5% CO 2 were grown and maintained in RPMI 1640 (Gibco) supplemented with 10% (v / v) FBS (Sigma) and 1% (v / v) penicillin / streptomycin. Primary human PBMCs were freshly isolated and cultured in supplemented with 10% (v / v) heat-inactivated FBS, 0.01% (v / v) 2-β-mercaptoethanol (Life Sciences) and 1% (v / v) L- Glutamine in RPMI 1640 (Gibco) at 37°C, 5% CO 2 down to maintain. CD34+ bone marrow-derived cells (HSCs) were purchased from Lonza,...
Embodiment 2
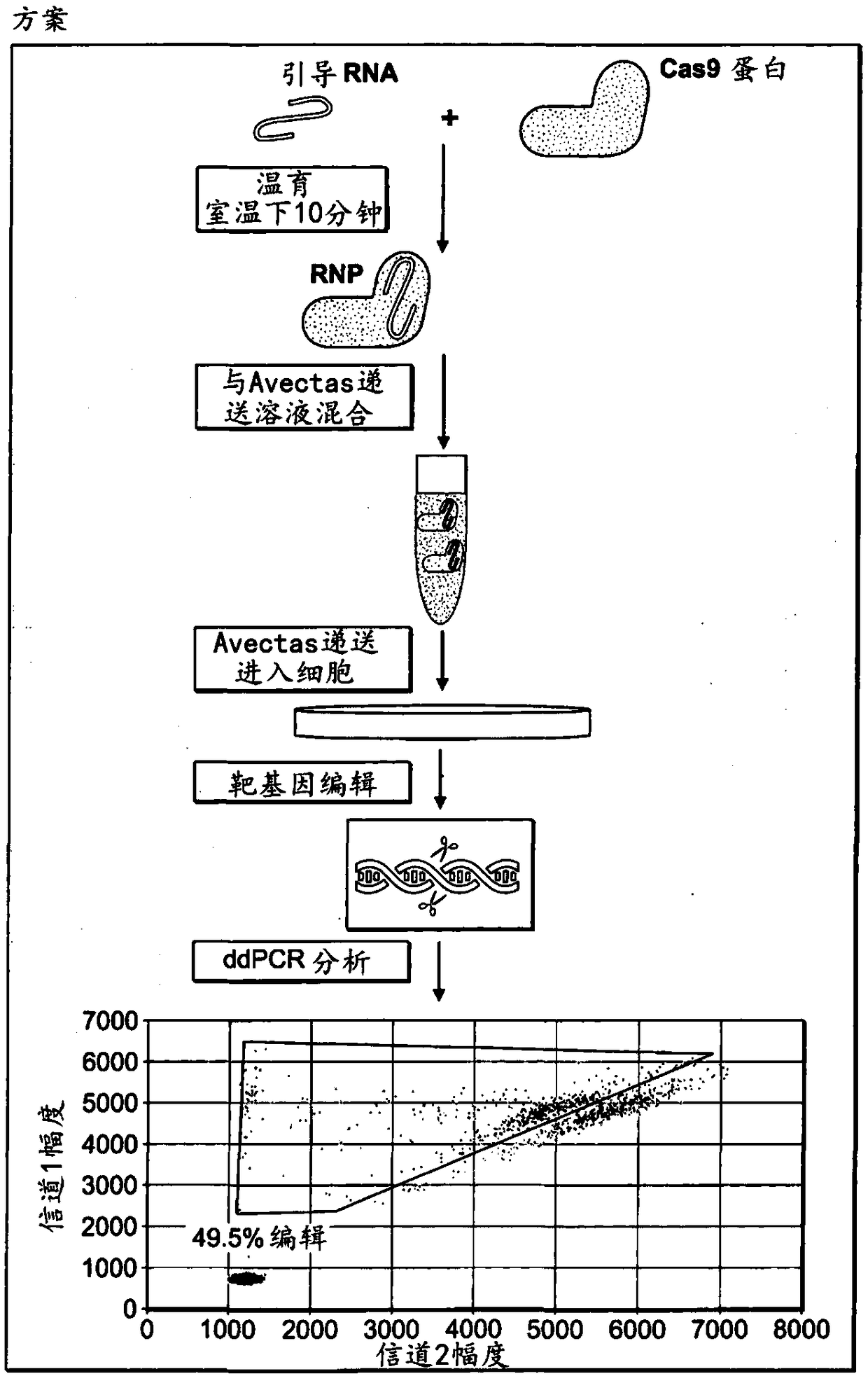
[0270] Example 2: Establishing and testing the function of the gene editing tool and the impact of the delivery solution on the function of the gene editing tool In vitro editing analysis of
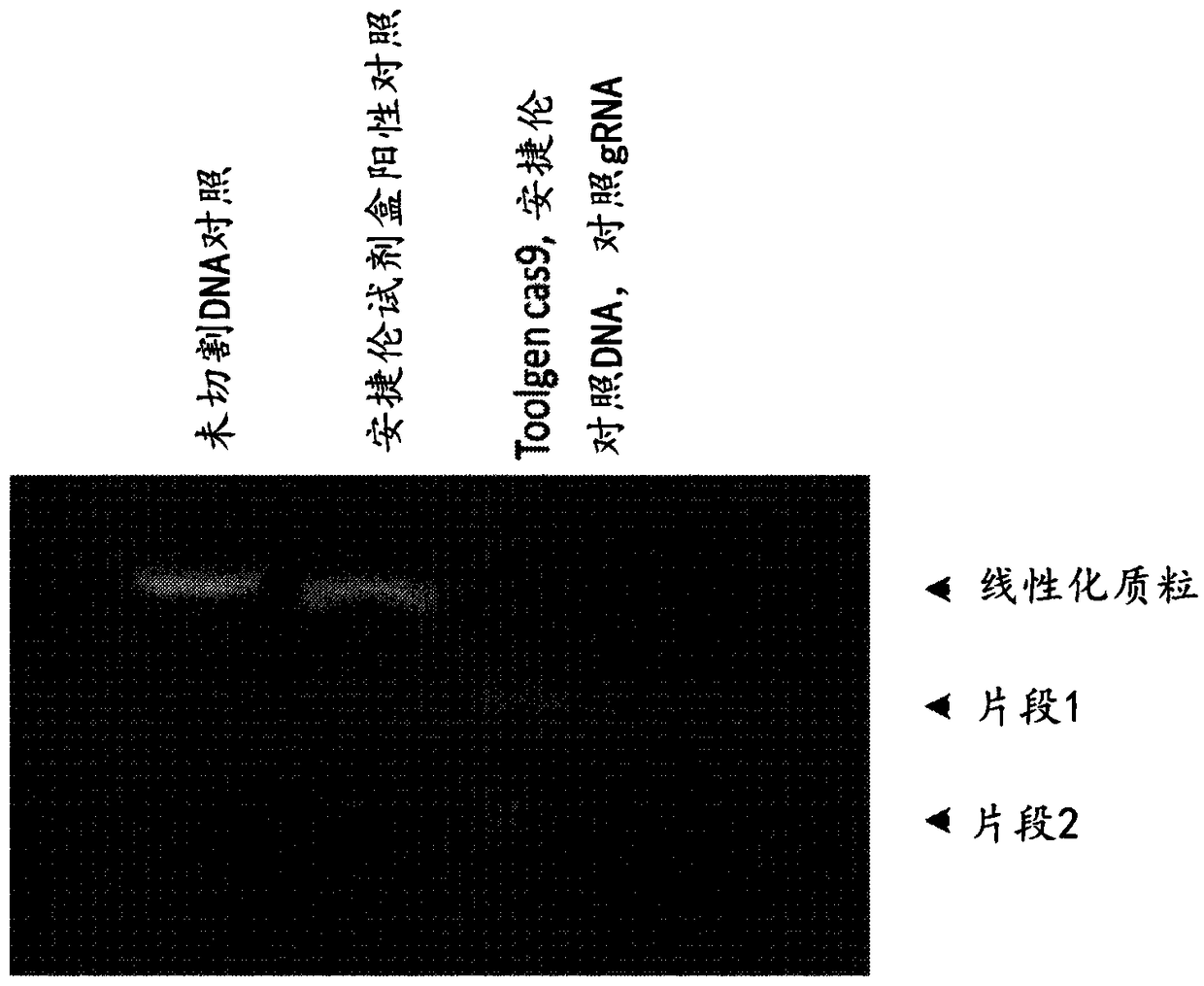
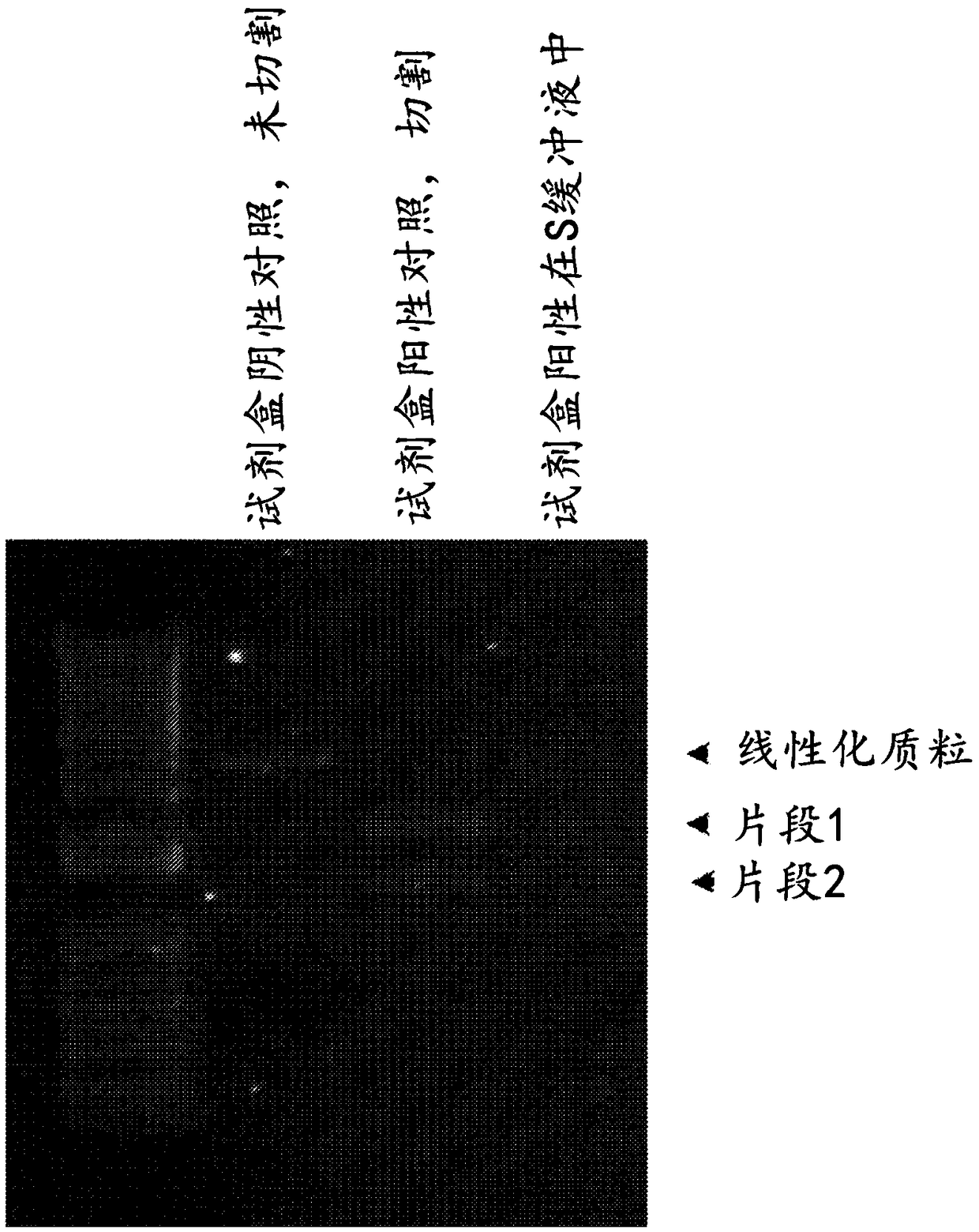
[0271] The Crispr Cas9 system for gene editing has been widely adopted. However, most users use the DNA plasmid form of Cas9 in conjunction with guide RNA. Systems that deliver forms of RNP are of interest as this is more transient and should therefore lead to fewer off-target effects. The necessary tools (recombinant Cas9 nuclease, single guide, two-part guide) have recently become commercially available. Custom guide RNA sequences were designed based on corresponding plasmid sequences reported in the literature. Perform a confirmatory analysis to check the quality of the reagents. To evaluate gene editing tools, an assay for in vitro editing tests based on the Agilent SureGuide Cas9 Programmable Nuclease Kit was developed. In this assay, Cas9 protein is incubated with a guide (...
Embodiment 3
[0293] Example 3: Conjugation of Cas9 to a fluorescent label for visualization of delivery into cells.
[0294] To utilize the delivery platform technology to analyze whether Cas9 enters the cell after delivery, conjugation of the protein with a fluorescent label was explored. Cas9 labeling methods targeting amine groups must be avoided as they lead to Cas9 precipitation due to the physicochemical properties of Cas9. Therefore, Cas9 was labeled with the fluorescent tag Alexa-488 maleimide via the sulfhydryl group of cysteine (2 cysteine residues per molecule of protein). This reaction did not alter the overall charge of the protein at physiological pH. The experimental protocol followed was based on previous work (Zuris, JA et al., Nat Biotechnol 2015; 33(1) 73-80).
[0295] Material
[0296] Cas9 was purchased from Toolgen Genome Engineering (Korea) or IDT Integrated DNA Technologies (USA). TCEP (tris(2-carboxyethyl)phosphine), Hepes, glycerol and KCl were of anal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com