Antibody nucleic acid drug conjugate with dual enzyme-sensitive properties, preparation method of conjugate and application of conjugate
A technology of nucleic acid drugs and conjugates, which can be used in medical preparations containing non-active ingredients, medical preparations containing active ingredients, and anti-tumor drugs, etc., and can solve problems such as insufficient cell uptake, low drug efficacy, and low drug concentration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
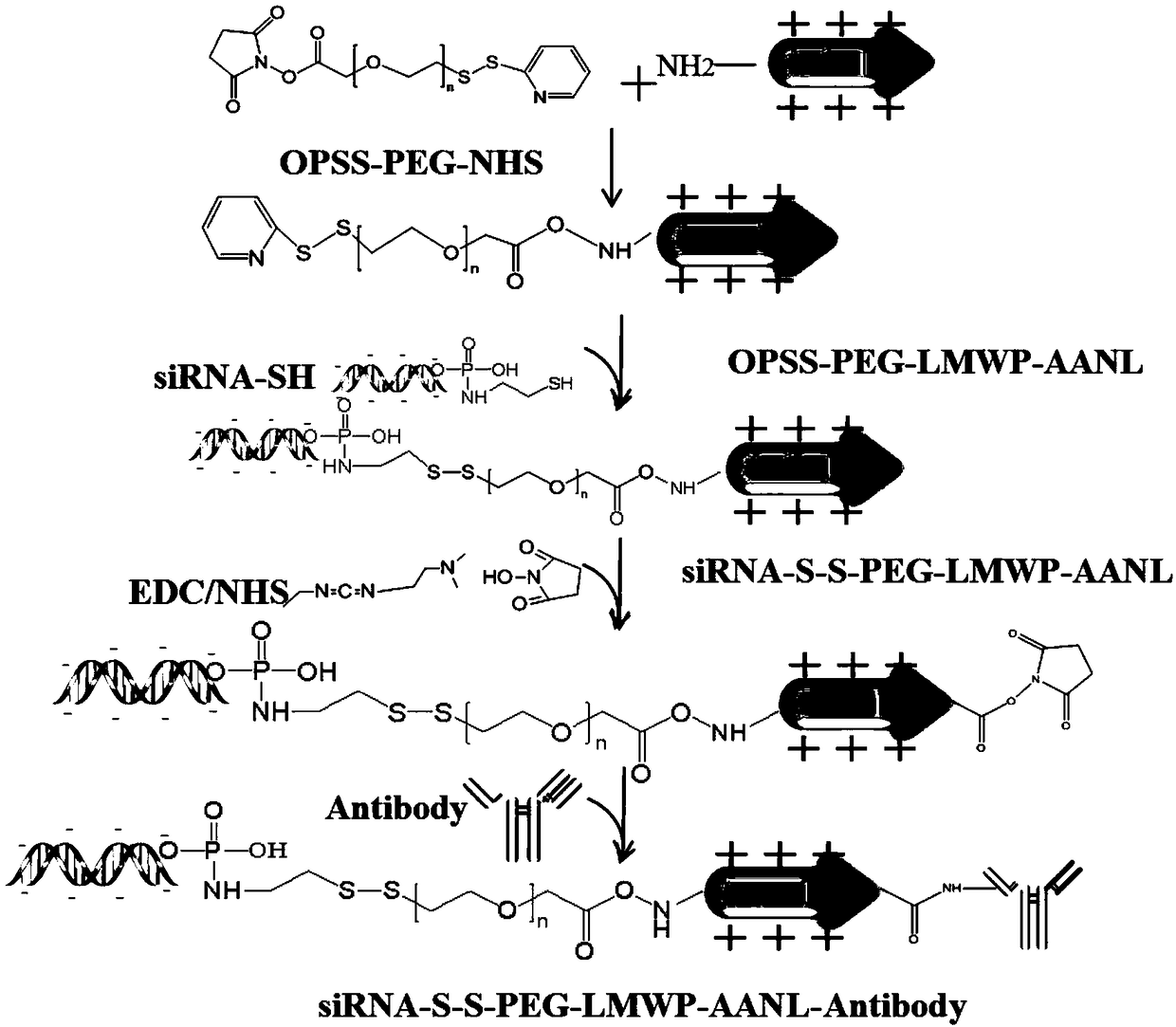
[0054] Synthesis of Legumain prodrug antibody targeting drug delivery system siRNA-LMWP-AANL-Antibody (siRNA-LMWP-AANL-cetuximab). Synthetic route such as figure 2 shown.
[0055] First prepare the fusion peptide-nucleic acid conjugate containing the penetrating peptide: take 10mg LMWP-AANL and dissolve it in an appropriate amount of buffer (20mM NaH 2 PO 4 , pH=6.9) was placed in a 20 mL vial, and 74 mg of OPSS-PEG-NHS (where n was 5) was weighed and dissolved in anhydrous DMSO, and added dropwise into the vial after it was completely dissolved. Place the vial in a shaker, react at 25° C. and 220 rpm for 6 h, and purify the reaction solution with a heparin column to obtain an OPSS-PEG-LMWP-AANL solution.
[0056] Take 5nmol of thiolated siRNA and dissolve it in an appropriate amount of buffer (20mM NaH 2 PO 4 , 1mM EDTA, pH=6.9), then quickly drop the OPSS-PEG-LMWP-AANL solution obtained above into the buffer containing siRNA, react at 220rpm at 40°C for 1h, and purify ...
Embodiment 2
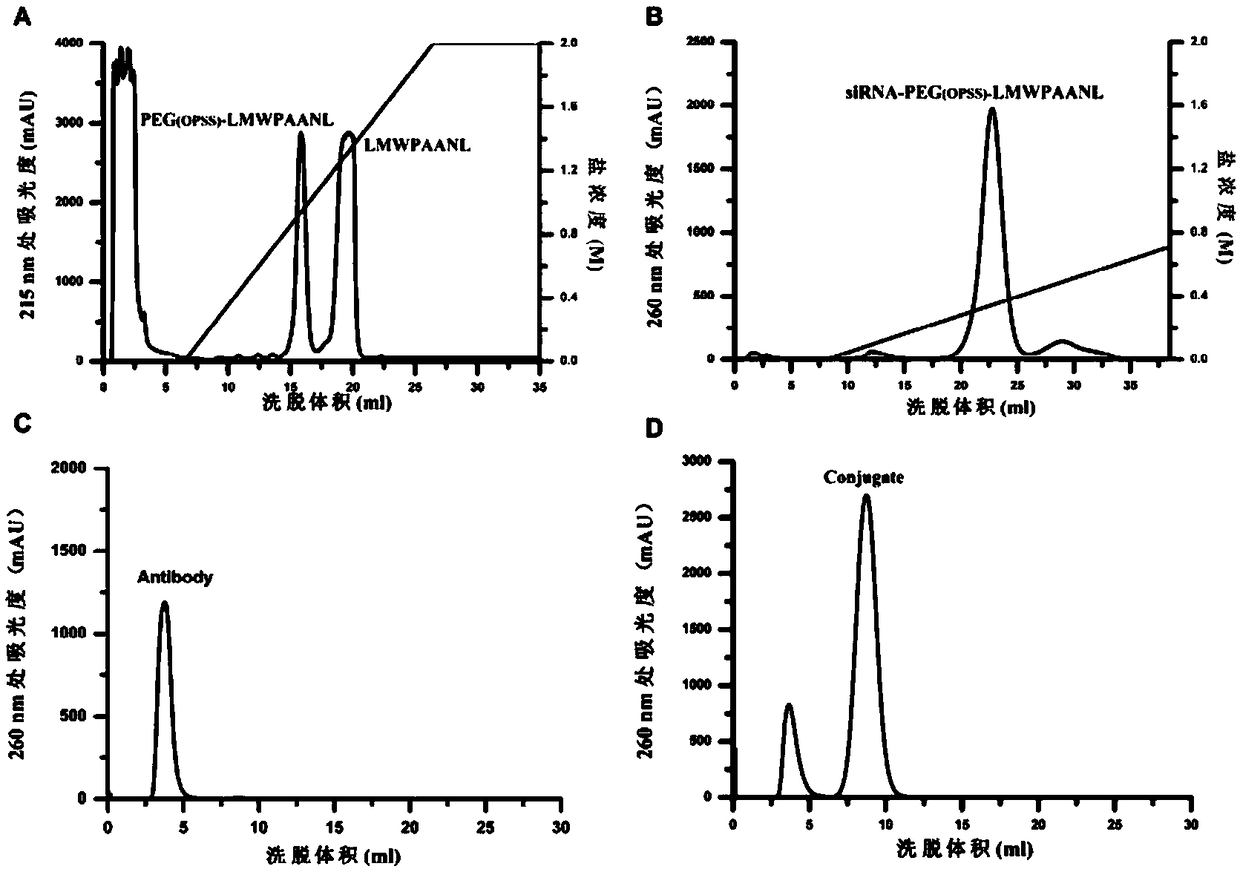
[0060] Purification of siRNA-LMWP-AANL-Antibody in Legumain Prodrug Antibody Targeted Delivery System.
[0061] After reacting LMWP-AANL with NHS-PEG-OPSS, the product PEG-LMWP-AANL was purified by a heparin column to remove unreacted LMWP-AANL. The low-salt mobile phase used in the purification process was 20mM NaH 2 PO 4 (pH=6.9), high salt mobile phase is 20mM NaH 2 PO 4 , 2M NaCl (pH=6.9). The PEG-LMWP-AANL compound was obtained by linear gradient elution and purification of 0-100% high-salt mobile phase under the conditions of a detection wavelength of 215nm and a flow rate of 1ml / min.
[0062] After PEG-LMWP-AANL was reacted with siRNA, the single product siRNA-PEG-LMWP-AANL was purified by anion exchange column DEAE column. The separation conditions are as follows: the low-salt mobile phase is 20mM NaH 2 PO 4 , 1mM EDTA, high salt mobile phase is 20mM NaH 2 PO 4 , 1 mM EDTA, 1 M NaCl. The detection wavelength is 260nm, and the flow rate is 1ml / min. Linear gra...
Embodiment 3
[0067] Characterization of Legumain Prodrug Antibody Targeted Drug Delivery System siRNA-LMWP-AANL-Antibody.
[0068] 3.1 Chromatographic detection
[0069] like Figure 4 As shown, after purification by desalting column, the reacted solution of siRNA-PEG-LMWP-AANL and Antibody eluted at about 3ml and 8ml respectively, while the physical mixture of siRNA-LMWP-AANL and Antibody only eluted at about 3ml And it is the same as the previous peak position in the previous sample. According to the above chromatographic behavior, it can be preliminarily judged that the synthetic product is siRNA-LMWP-AANL-Antibody system.
[0070] 3.2 Agarose gel electrophoresis detection
[0071] With the support of the above chromatographic results, the synthetic product was further characterized by agarose gel electrophoresis. The result is as Figure 5 As shown, compared with siRNA, siRNA-PEG-LMWP-AANL has less negative charges exposed due to the linking of positively charged LMWP-AANL, and th...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap