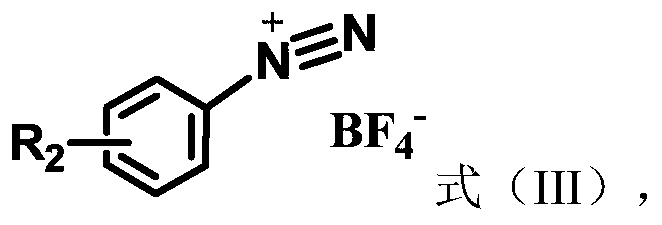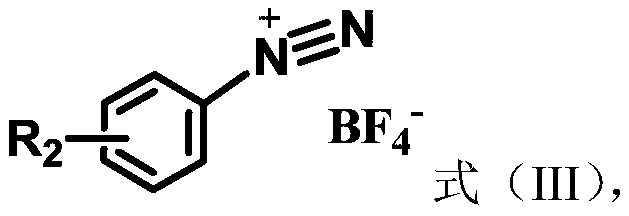A kind of preparation method of sulfoxide compound
A compound and sulfoxide technology, which is applied in the field of preparation of sulfoxide compounds, can solve the problems of difficult preparation of catalysts, poor reaction selectivity, harsh oxidation conditions, etc., and achieve the effects of low cost, mild conditions and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
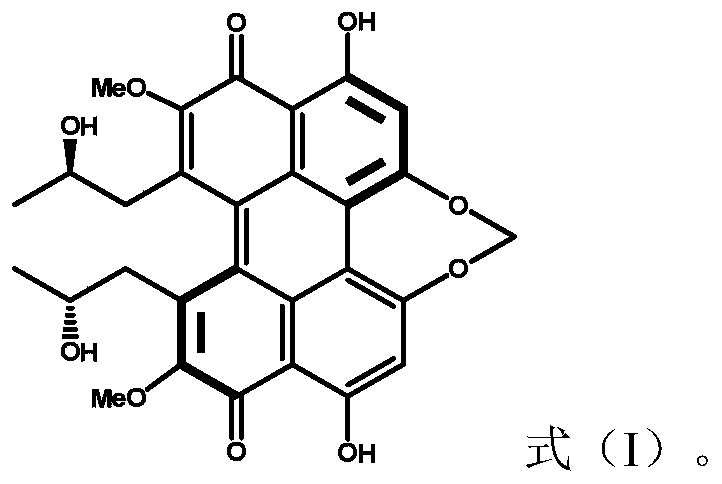
[0032] Embodiment 1. Catalyzed synthesis of 1-bromo-4-(4-toluenesulfinyl)benzene by cercosporin catalyst
[0033] Add cercosporin (0.005mmol), p-bromobenzenetetrafluoroborate diazonium salt (0.5mmol), p-methylthiophenol (0.5mmol), 2mL chloroform, and 120 microliters of pyridine in sequence in a 10mL reaction tube, Then oxygen protection, 15W white light irradiation, room temperature 25 degrees Celsius reaction 24h. The reaction solution was washed three times with water, and the organic phase was collected and then dried over anhydrous magnesium sulfate. After filtration, the solvent was evaporated to dryness by rotary evaporation, and the thin-layer silica gel plate of 300-500 mesh was used for rapid separation, and the eluent used was ethyl acetate / petroleum ether (v:v=1:10) to obtain 1-bromo-4-(4 -toluenesulfinyl)benzene, yield 70%.
Embodiment 2
[0034] Embodiment 2. Catalyzed synthesis of 1-chloro-4-(4-toluenesulfinyl)benzene by cercosporin catalyst
[0035] Add cercosporin (0.005mmol), p-chlorobenzenetetrafluoroborate diazonium salt (0.5mmol), p-methylthiophenol (0.5mmol), 2mL chloroform, and 120 microliters of pyridine successively into a 10mL reaction tube, Then oxygen protection, 15W white light irradiation, room temperature 25 degrees Celsius reaction 24h. The reaction solution was washed three times with water, and the organic phase was collected and then dried over anhydrous magnesium sulfate. Filtrate, dry the solvent by rotary evaporation, and use a 300-500 mesh thin-layer silica gel plate to separate quickly, and the eluent used is ethyl acetate / petroleum ether (v:v=1:10) to obtain 1-chloro-4-(4 -toluenesulfinyl)benzene, yield 70%.
[0036] Embodiment 2. Catalyzed synthesis of 1-nitro-4-(4-toluenesulfinyl)benzene by cercosporin catalyst
[0037] Add cercosporin (0.005mmol), p-nitrobenzenetetrafluoroborate...
Embodiment 4
[0038] Embodiment 4. Catalyzed synthesis of 1-formaldehyde-4-(4-toluenesulfinyl)benzene by cercosporin catalyst
[0039] Add cercosporin (0.005mmol), p-formaldehyde benzenetetrafluoroborate diazonium salt (0.5mmol), p-methylthiophenol (0.5mmol), 2mL chloroform, and 120 microliters of pyridine in sequence in a 10mL reaction tube. Then oxygen protection, 15W white light irradiation, room temperature 25 degrees Celsius reaction 24h. The reaction solution was washed three times with water, and the organic phase was collected and then dried over anhydrous magnesium sulfate. Filtrate, dry the solvent by rotary evaporation, and use a 300-500 mesh thin-layer silica gel plate to separate quickly, and the eluent used is ethyl acetate / petroleum ether (v:v=1:10) to obtain 1-formaldehyde-4-(4 -toluenesulfinyl)benzene, yield 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com