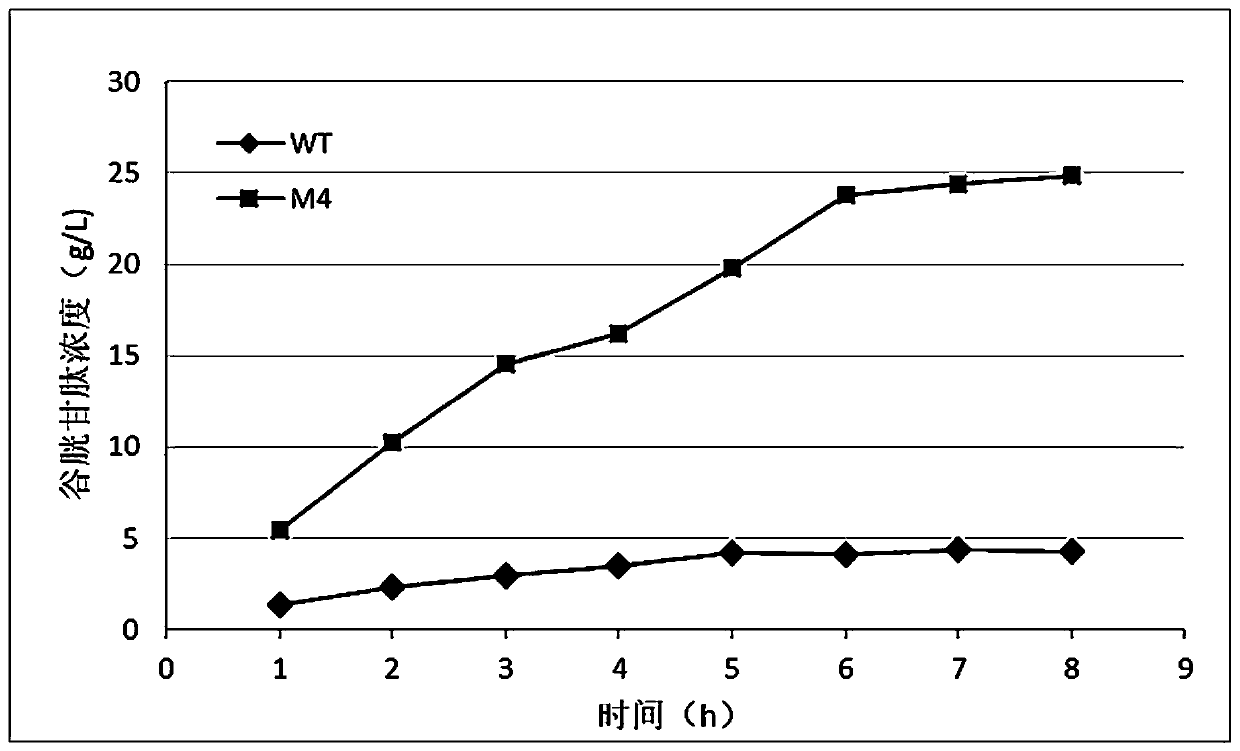
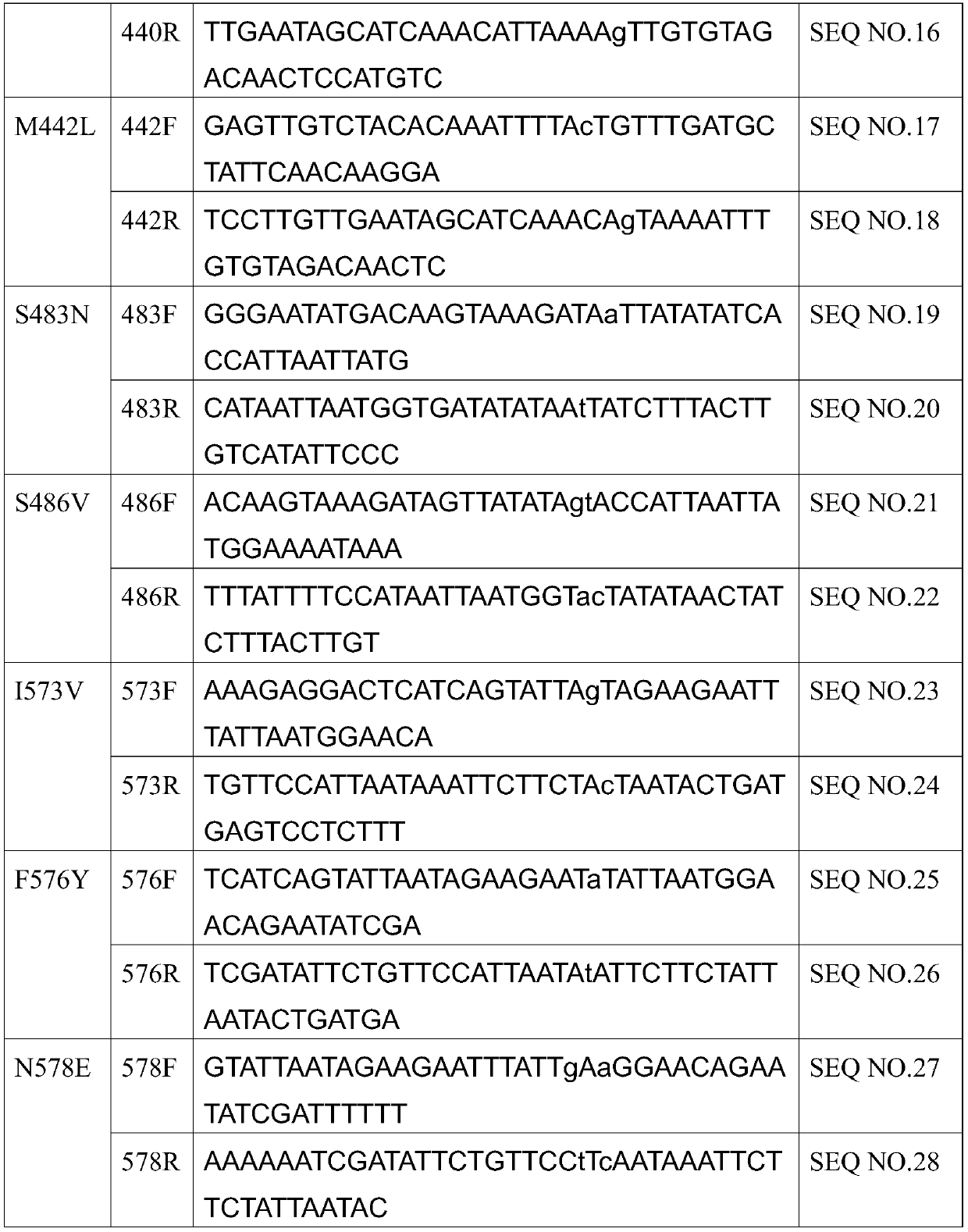
Mutants of bifunctional glutathione synthase and their application in glutathione synthesis
A glutathione and synthetase technology, applied in the direction of peptides, enzymes, ligases, etc., can solve the problems of low synthetase activity, low product tolerance, insufficient stability, etc., to increase product concentration and activity , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1, the construction of wild-type gdhF expression vector
[0042] According to the coding sequence of Bacillus cereus bifunctional glutathione synthetase in the GenBank database (GenBank: ACLS01000213.1, SEQ NO.2), Suzhou Jinweizhi Biotechnology Co., Ltd. was entrusted to synthesize the gene fragment and named it gshFbc gene. In order to facilitate subsequent cloning, two bases of CC were added to the 5' end of the gene fragment to form a NcoI restriction site, and six bases of GGATCC were added to the 3' end to form a BamHI site.
[0043] The synthesized gshFbc gene and pET28a vector were double-digested with NcoI and BamHI, respectively, and the fragments were recovered with DNA gel kits (purchased from AXYGEN Company), respectively. After ligation with T4 DNA ligase, E. coli DH5α was transformed, and Kana Mycin-resistant LB plate screening to obtain clones.
[0044] Pick 8 clones, use a plasmid extraction kit (purchased from AXYGEN) to extract the plasmids...
Embodiment 2
[0045] Embodiment 2, the expression of GshFbc and the preparation of enzyme
[0046] The expression vector pET28a-gshFbc obtained in Example 1 was transformed into Escherichia coli BL21(DE3) to obtain a genetically engineered Escherichia coli strain capable of highly expressing glutathione synthetase.
[0047] The engineering bacteria highly expressing glutathione synthase were fermented and cultivated in shake flasks according to conventional methods, and when the OD600 of the fermentation broth reached 0.6-0.8, adding a final concentration of 1 mM isopropylthiogalactoside (IPTG) induced , lower the temperature to 28 degrees and continue to cultivate for about 16 hours, centrifuge at 4000 rpm for 20 minutes, collect the bacteria, wash twice with 50mM PBS buffer solution of pH7.4, and obtain the bacteria containing glutathione synthase for subsequent catalysis Research and Enzyme Activity Assays.
Embodiment 3
[0048] Embodiment 3, the mensuration of glutathione synthetase activity
[0049] Weigh 10 g of the collected thalli containing glutathione synthase, and add cetyltrimethylammonium bromide (CTAB) with a final concentration of 0.5% to permeabilize the cells, add 100 mM PBS with pH 8.0 Buffer to a final volume of 50mL to obtain glutathione synthase crude enzyme solution
[0050] In the 5mL reaction system, add L-glutamic acid, L-cysteine, L-glycine and ATP to a final concentration of 50mM, add magnesium ions to a final concentration of 10mM, add 500uL of crude enzyme solution, and add 100mM PBS with pH8.0 buffer to a final volume of 5 mL to obtain a reaction solution.
[0051] The reaction solution was reacted at 37°C for 30 minutes, terminated in a boiling water bath for 5 minutes, centrifuged at 4000 rpm for 20 minutes, and the supernatant was collected for subsequent detection.
[0052] Take 250uL of the supernatant sample, add 750uL of 0.2M sodium hydroxide solution, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com