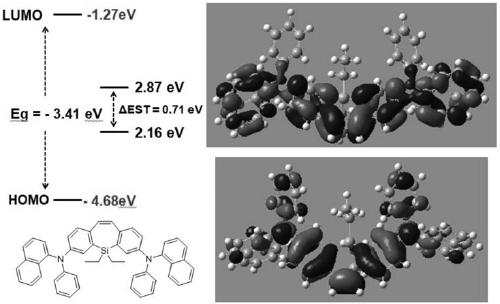
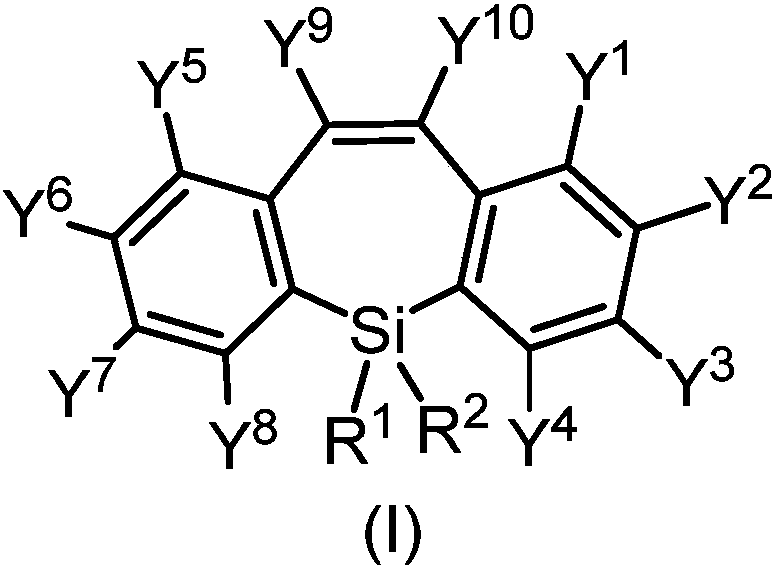
Dibenzo-heterocyclic compound and preparation method and applications thereof
一种杂环化合物、二苯的技术,应用在有机电致发光材料领域,能够解决使用寿命缩短、器件发光效率降低、热稳定性低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] This implementation provides a dibenzoheterocyclic compound, which has the structure shown in the following formula SP-01:
[0067]
[0068] The synthetic route of the dibenzoheterocyclic compound shown in formula SP-01 is as follows:
[0069]
[0070] The preparation method of the dibenzoheterocyclic compound shown in formula SP-01 comprises the following steps:
[0071] 1. Preparation of intermediate 1-1
[0072] Get 500 milliliters double-necked round-bottom flasks and put into stirrer bar and connect reflux pipe, fill with nitrogen after drying; First add compound A-1 (20.548 grams, 1.0 equivalent), NBS (N-bromosuccinimide , 19.578 grams, 1.1 equivalents), and AIBN (azobisisobutyronitrile, 0.821 grams, 0.5 mole percent), then add carbon tetrachloride (250 milliliters) and stir and mix for 10 minutes, and finally heat to reflux and react 24 hours; after it was warmed up, water (200 ml) was added, followed by extraction with ethyl acetate (3×200 ml), the resul...
Embodiment 2
[0083] This implementation provides a dibenzoheterocyclic compound, which has the structure shown in the following formula SP-02:
[0084]
[0085] The synthetic route of the dibenzoheterocyclic compound shown in formula SP-02 is as follows:
[0086]
[0087] The preparation method of the dibenzoheterocyclic compound shown in formula SP-02 comprises the following steps:
[0088] 1. Preparation of intermediate 1-2
[0089] Get 500 milliliters double-necked round-bottom flasks and put into stirrer bar and connect reflux tube, fill with nitrogen after drying; First add compound A-2 (20.548 grams, 1.0 equivalent), NBS (N-bromosuccinimide , 19.578 grams, 1.1 equivalents), and AIBN (azobisisobutyronitrile, 0.821 grams, 0.5 mole percent), then add carbon tetrachloride (250 milliliters) and stir and mix for 10 minutes, and finally heat to reflux and react 24 hours; after it was warmed up, water (200 ml) was added, followed by extraction with ethyl acetate (3×200 ml), the resul...
Embodiment 3
[0100] This implementation provides a dibenzoheterocyclic compound, which has the structure shown in the following formula SP-03:
[0101]
[0102] The synthetic route of the dibenzoheterocyclic compound shown in formula SP-03 is as follows:
[0103]
[0104] The preparation method of the dibenzoheterocyclic compound shown in formula SP-03 comprises the following steps:
[0105] 1. Preparation of Intermediates 1-3
[0106] Get 500 milliliters double-neck round-bottom flasks and put into stirrer bar and upper connection reflux tube, fill with nitrogen after drying; First add compound A-3 (20.548 grams, 1.0 equivalent), NBS (N-bromosuccinimide , 19.578 grams, 1.1 equivalents), and AIBN (azobisisobutyronitrile, 0.821 grams, 0.5 mole percent), then add carbon tetrachloride (250 milliliters) and stir and mix for 10 minutes, and finally heat to reflux and react 24 hours; after it was warmed up, water (200 ml) was added, followed by extraction with ethyl acetate (3×200 ml), t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| internal quantum efficiency | aaaaa | aaaaa |
| process yield | aaaaa | aaaaa |
| process yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com