Application of thiazole benzamide derivatives in the preparation of anti-osteoporosis and anti-osteoarthritis drugs
A technology for benzamide and osteoarthritis, applied in the field of pharmaceutical use, can solve the problems of not being able to significantly reduce the risk of atypical fractures, failing to meet anti-osteoporosis treatment, failing to meet treatment needs, etc., achieving novel structure, Simple structure and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: synthesis of thiazole benzamide derivatives
[0047]
Embodiment 2
[0048] Example 2: NF-κB signaling pathway inhibition experiment
[0049] S1. Cell culture.
[0050]RAW264.7 cells stably transfected with high expression of NF-κB were cultured in vitro. DMEM high-glucose medium containing 10% fetal bovine serum was used for routine maintenance and passage at 37°C and 5% carbon dioxide concentration.
[0051] S2. Compound intervention.
[0052] Collect logarithmic phase cells and make the cell suspension concentration 5×10 5 cells / ml, added to 96-well cell culture plate. After culturing in a carbon dioxide incubator for 24 hours, the culture medium was replaced with media containing different compound concentrations, and co-cultivated for 6 hours. The compounds to be tested were prepared as solutions at different concentrations using DMSO. Three parallel wells were set up for each concentration, and a control group without compound treatment was set up for comparison.
[0053] S3. Test method.
[0054] After the experiment, the cells we...
Embodiment 3
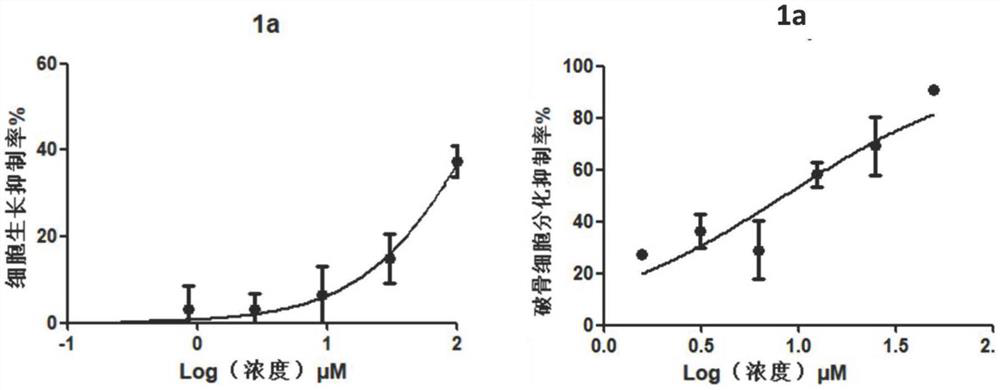
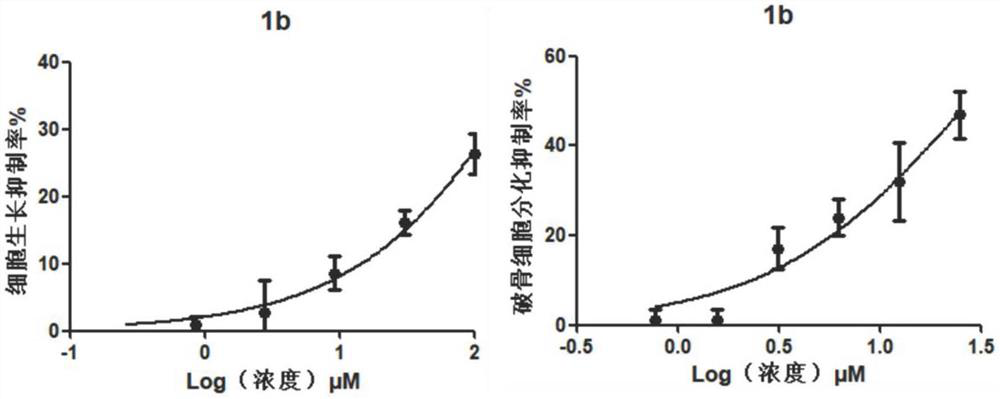
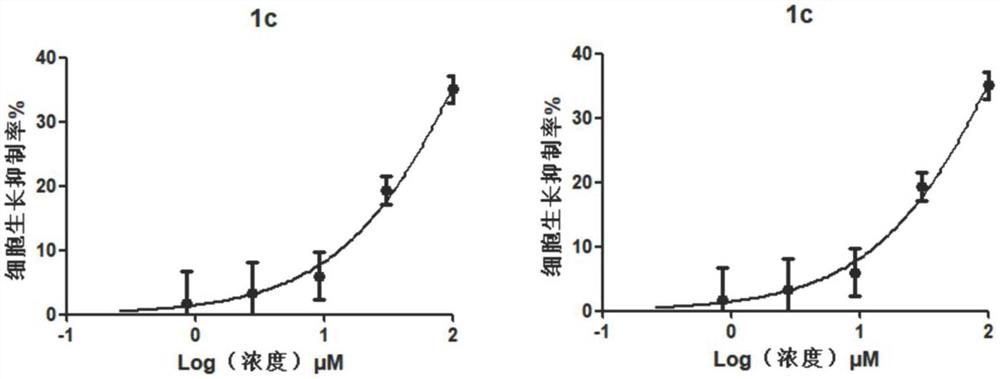
[0059] Example 3: Toxicity assay of compound cells
[0060] S1. Cell culture.
[0061] RAW264.7 cells were cultured in vitro. DMEM high-glucose medium containing 10% fetal bovine serum was used for routine maintenance and passage at 37°C and 5% carbon dioxide concentration.
[0062] S2. Compound intervention.
[0063] Collect logarithmic phase cells and make the cell suspension concentration 1×10 5 cells / ml, added to 96-well cell culture plate. After culturing in a carbon dioxide incubator for 24 hours, the culture solution was replaced with a medium containing different compound concentrations, and the culture was continued for 2 days, and the cytotoxicity was detected on the 3rd day. The compounds to be tested were prepared as solutions at different concentrations using DMSO. Three parallel wells were set up for each concentration, and a control group without compound treatment was set up for comparison.
[0064] S3. Test method.
[0065] MTT [3-(4,5-dimethylthiazole-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com