A group of gene recombinant human corticotropin precursors for improving serum glucocorticoid levels and preparation method
A technology of glucocorticoid and gene recombination, applied in adrenocorticotropic hormone, chemical instruments and methods, recombinant DNA technology, etc., can solve the problems of transmission of animal viruses and mycoplasma, and high production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
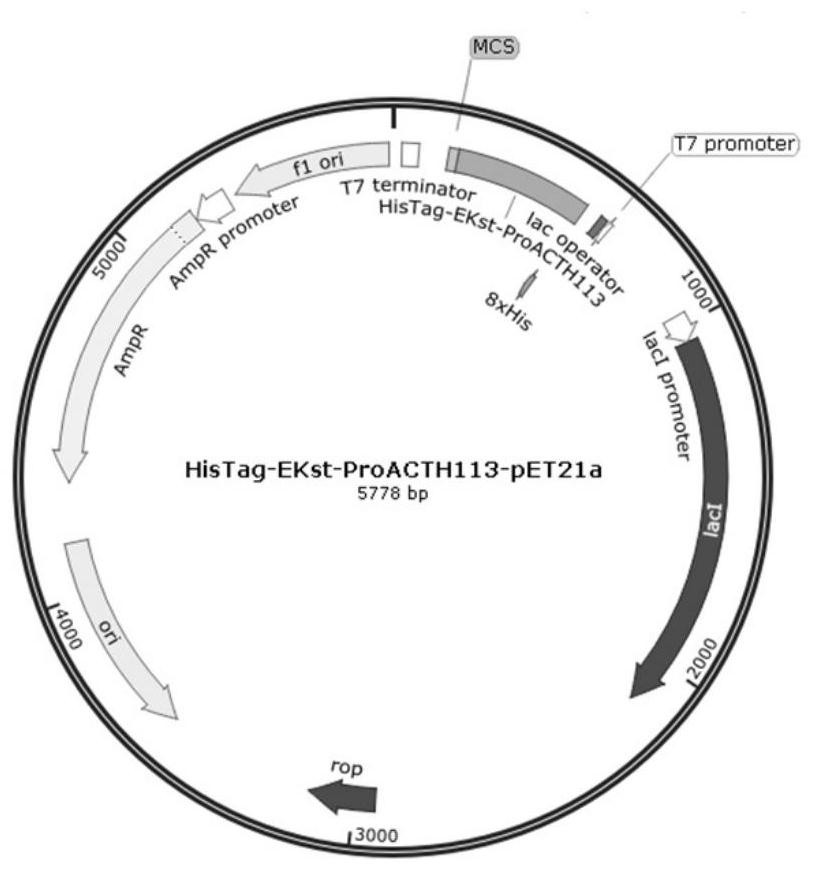
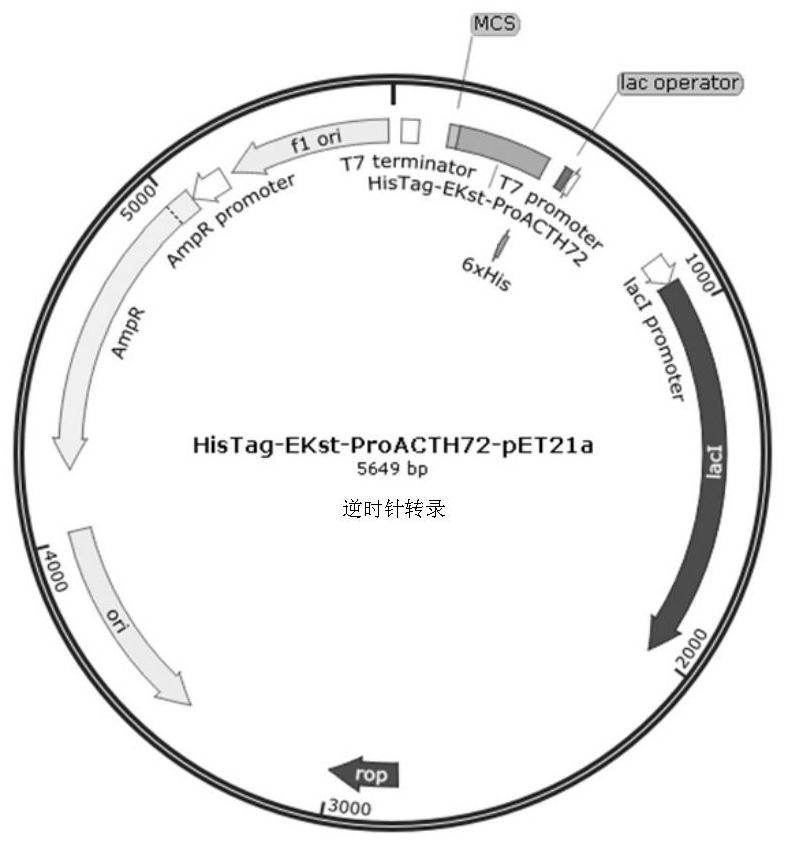
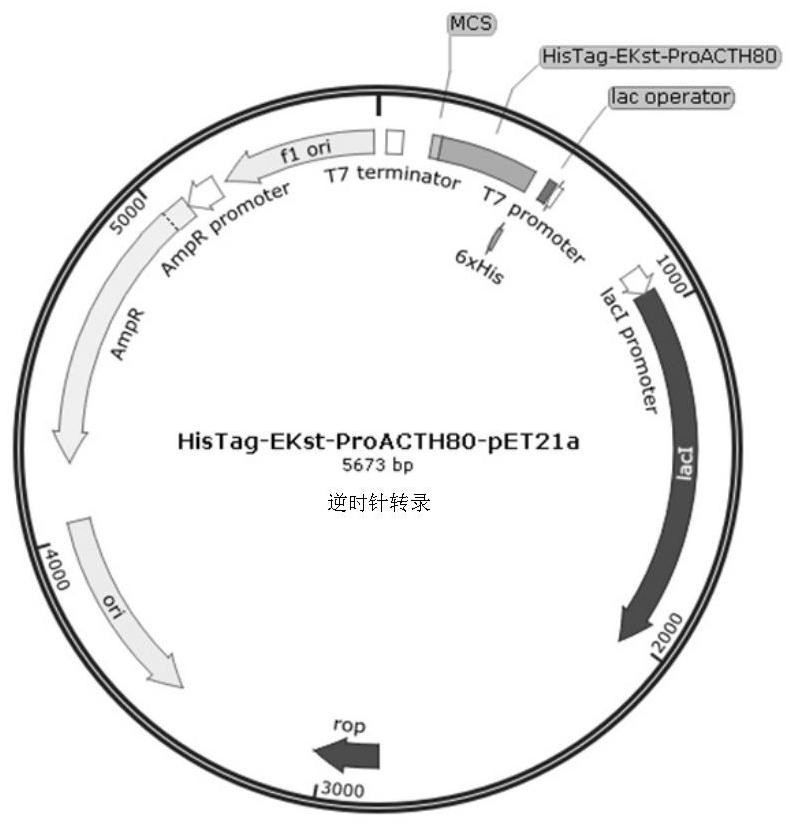
[0056] Embodiment 1: This embodiment expresses, isolates and purifies the recombinant ProACTH113, ProACTH72, ProACTH80 and ProACTH141 fusion proteins, and performs the following steps:
[0057] 1. The ProACTH141CDS sequence was optimized and designed according to the codon bias in the prokaryotic expression system to be suitable for high-efficiency expression in the E. coli expression strain BL21-DE3. The fusion protein prokaryotic expression sequence NdeI-HisTag-EKst-ProACTH141-SalI with His tag and enterokinase cleavage site was constructed using NdeI and SalI as the restriction sites at the upstream and downstream ends of the CDS sequence of the fusion expression protein. This sequence will serve as a template for PCR amplification of the ProACTH113, ProACTH72 and ProACTH80 sequences. Specific steps are as follows:
[0058] Primer design: using the above optimized NdeI-HisTag-EKst-ProACTH141-SalI sequence as a template, design the upstream primers NdeI-HisTag-EKst-ProACTH1...
specific Embodiment approach 2
[0068] Embodiment 2: The difference between this embodiment and Embodiment 1 is that the pET21a plasmid is not used, but pET30a is used as the expression vector.
specific Embodiment approach 3
[0069] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is that pET30a or pET21a plasmid is not used, but pET28a is used as the expression vector.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com