An injection composition comprising hyaluronidase for removal of topical fat
An injection composition, hyaluronidase technology, applied in the direction of enzymes, hydrolytic enzymes, glycosylases, etc., can solve problems such as unavoidable side effects, achieve skin lifting effect, slim and smooth skin regeneration, skin elasticity Keep the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0072] [Example] Preparation of injection composition
[0073] ingredient
[0074] For hyaluronidase, Liporase Inj. (1500IU / vial, including 13.3mg lactose hydrate) of Daehan New Pharm Co., Ltd was used, in which lidocaine (Hanmi Pharm. Co., Ltd.), pheniramine (Yuhan Co.), L-carnitine (Dream Pharma), and vitamin C (Daewoo Pharm Co., Ltd.), and the mixture was dissolved in a saline solution to prepare a 100cc injection solution composition .
Example Embodiment
[0075] [Example 1]
[0076] Lipolysis of the injection composition of the present invention
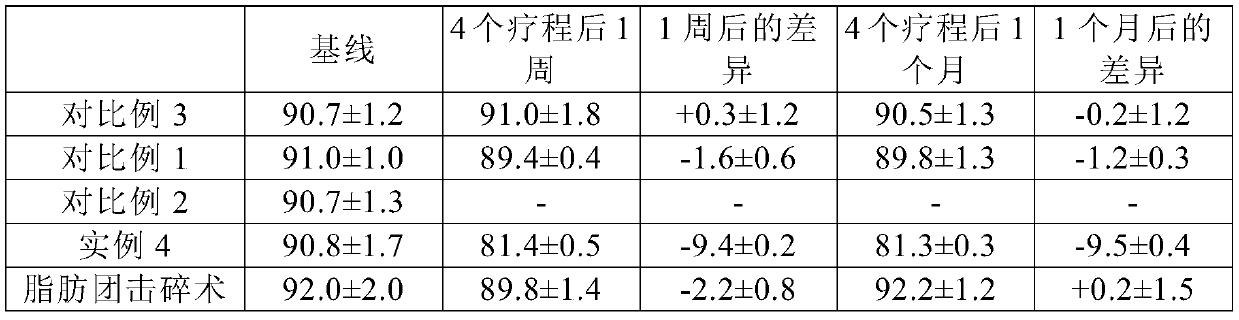
[0077] The changes in waist circumference after application of the injection composition of Example 4 and the injection compositions of Comparative Examples 1 to 3 and after cellulite endomology treatment for edema were measured and presented in Table 2.
[0078] The subjects of treatment were 40 patients (20 males, 20 females) with abdominal obesity and a BMI of 25 or higher, selected from adult outpatients aged 20 or above.
[0079] In the abdominal region between the ribs and the pubic bone, 1 cc of the injection composition was injected at 50 points, which were arranged at intervals of 1 cm from the belly button. Repeat the above injection every week for four weeks, each time you need to move the injection point. The reduction in waist circumference was measured 1 week after the completion of the treatment, and then measured again 1 month after the completion of the treatment. The aver...
Example Embodiment
[0090] [Example 2]
[0091] Evaluation of the side effects of the injection composition of the present invention
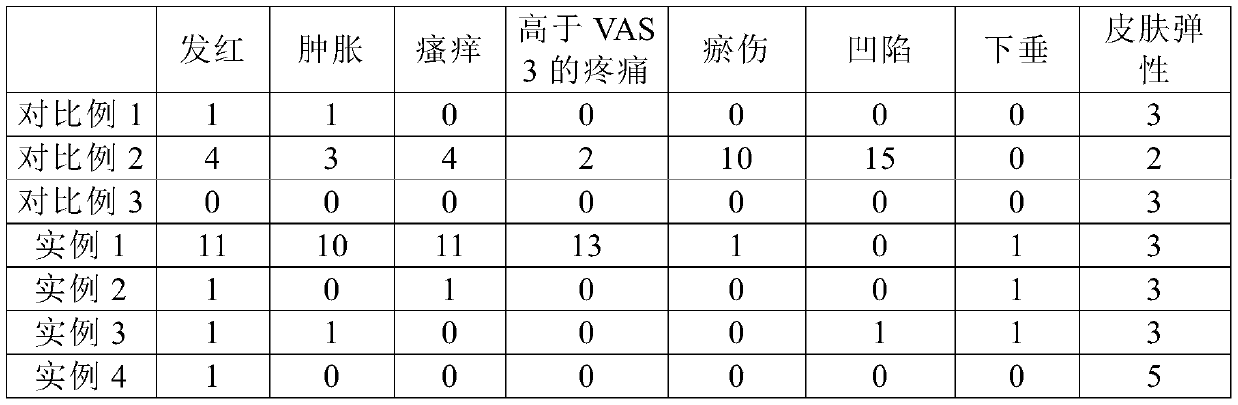
[0092] The injection compositions of Examples 1 to 4 and the injection compositions of Comparative Examples 1 to 3 were administered as in Example 1, and the side effects observed in each patient in the clinical trial are shown in Table 3.
[0093] [table 3]
[0094]
[0095] *Skin elasticity is determined by tactile evaluation using a 5-point scale: 1: very poor, 2: poor, 3: fair, 4: good, 5: very good.
[0096] As shown in Table 3, using the injection composition according to Example 1 of the present invention without a local anesthetic or antihistamine, compared with the high-dose composition of Comparative Example 2, side effects (for example, bruising or depression) Significant reduction, but pain and allergic reactions were observed. Compared with Example 1 or Comparative Example 2, the compositions of Examples 2, 3, and 4 containing local anesthetics and antihist...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com