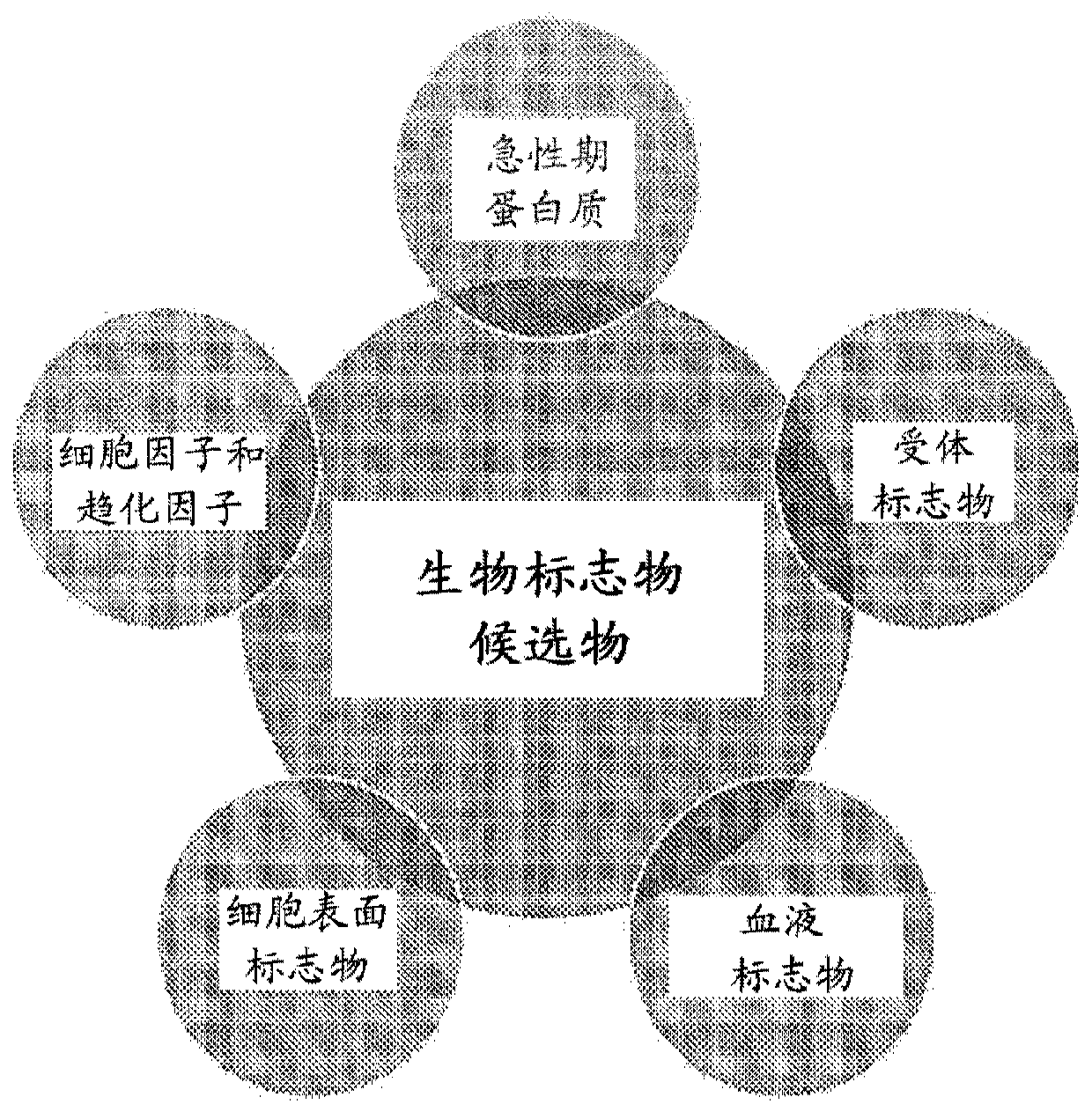
Estimating cellular populations
A neutrophil and sample technology, applied in the field of biomarker expression level, can solve the problem of unsatisfactory sensitivity of sepsis markers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0258] Development of a point-of-care (POC) alternative to flow cytometry-based neutrophil activation assays. Immunoassay cutoffs were assessed using the CD64 to NNM ratio.
[0259] Recognizing that a POC test for measuring neutrophil activation (nCD64i) would be of great clinical utility, a novel method was devised as described herein that would allow the use of algorithms and independent adaptation to the POC measurement. Values of the combination of markers were used to measure similar or equivalent values of flow cytometry nCD64i values.
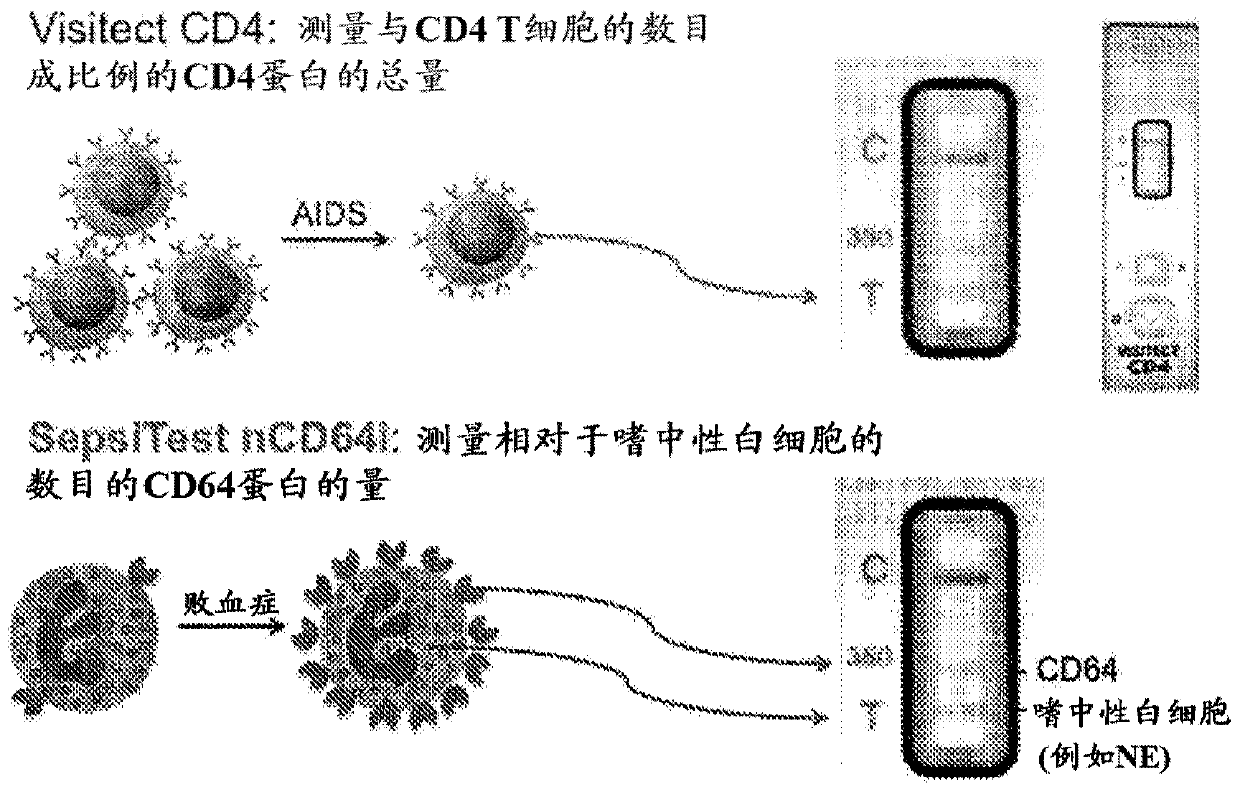
[0260] In some aspects, the methods of the invention take advantage of some of the inventors' experience in developing a lateral flow POC assay for measuring CD4 T cells (US Patent 8,409,818 and others).
[0261] According to the inventor's experience with CD4 POC test, it is known that the amount of CD4 per cell is constant, so the amount of CD4 is directly proportional to the number of CD4 T cells. In contrast, the amount of CD64...
Embodiment 2
[0277] Further developments, and in particular the utility of total CD64 levels and total NNM levels (ie internal and external levels) and different cut-off value determination methods in the diagnosis of sepsis are described.
[0278] In (FIG. 4C), it is illustrated that the amount of CD64 compared to NE (nCD64i measured by ELISA) is higher in samples with lower neutrophil / granulocyte counts, while novelly demonstrating the need for CD64i Variable cutoff value as a function of NE value to improve the use of CD64 to diagnose sepsis (see also Figure 5). It is worth noting that the results in that experiment were obtained using whole blood that had been monocyte / macrophage depleted using magnetic beads.
[0279] In further work these observations have been extended and a simplified method is described that only requires the use of NE and CD64 values from monocyte / macrophage undepleted whole blood. Monocyte / macrophage depletion is an optional step, especially for those with lo...
Embodiment 3
[0289] To better understand the utility of the assays described herein, septic patients and a subset of healthy subjects were examined using the commercial Leuko64 assay, which measures cell surface expression of CD64 on neutrophils by flow cytometry.
[0290] The Leuko64 kit [see e.g. Beckman Coulter - see U.S. Patent Publication No. 2013 / 0230867 filed in the name of Trillium Diagnostics LLC] contains three species specific for surface CD64 (FITC conjugated) and surface CD163 (phycoerythrin conjugated). A mixture of monoclonal antibodies and a proprietary fluorescent bead suspension for instrument calibration and normalization of leukocyte CD64 and CD163 expression on human blood leukocytes. The kit also contains erythrocyte lysis solution concentrate. Variations of this kit are available, such as the Accellix CD64 cassette and reader (LeukoDx).
[0291] like Figure 13As shown in , Leuko64 showed highly significantly higher values for sepsis patients compared to healthy ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com