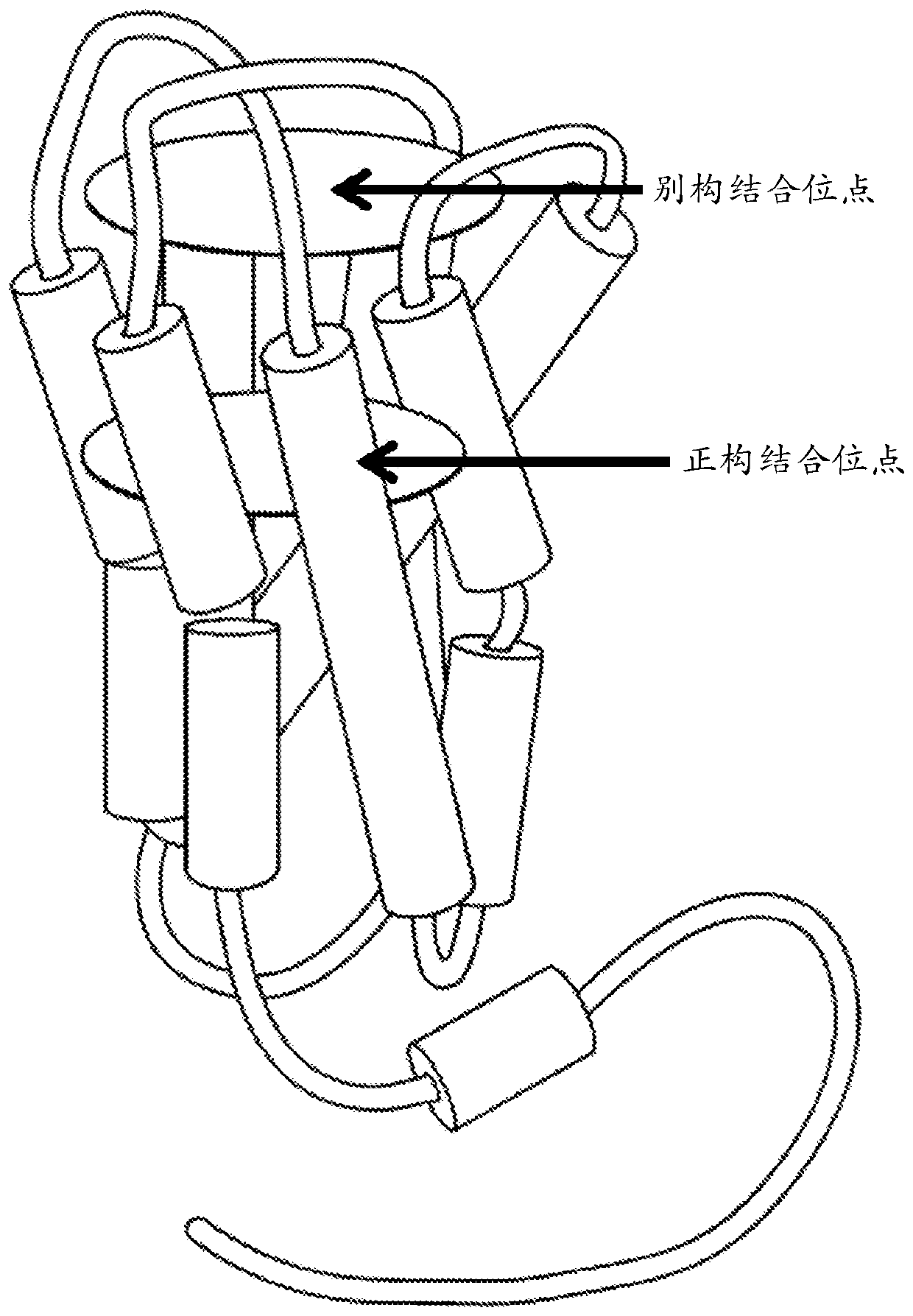
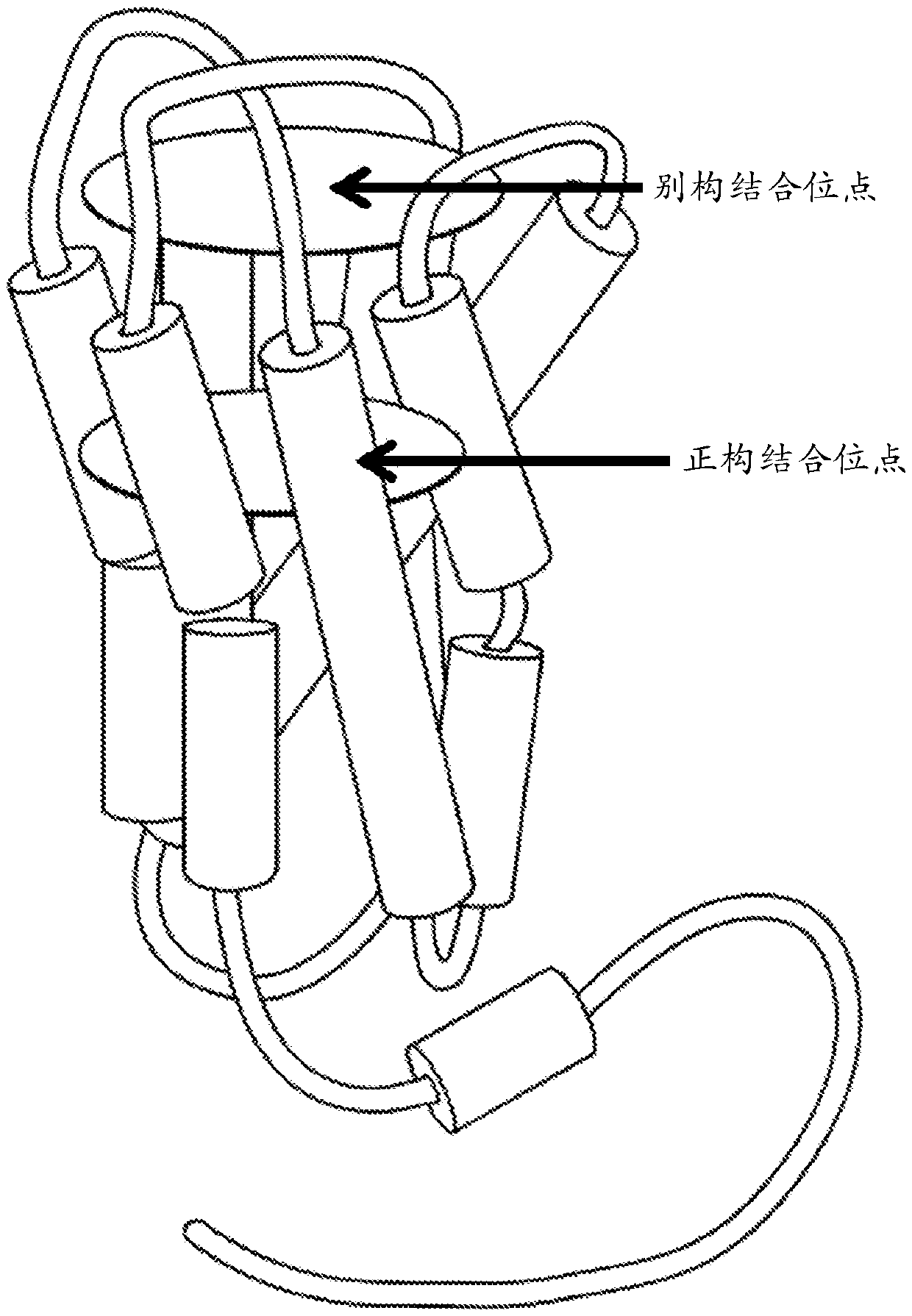
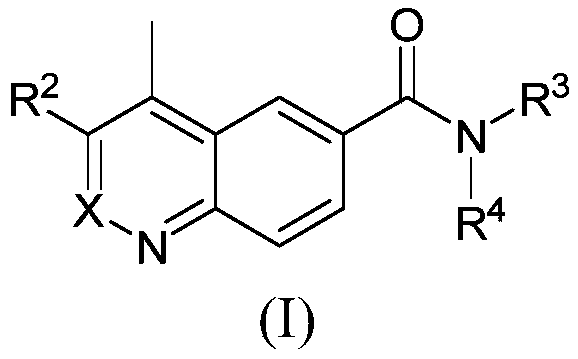
Positive allosteric modulators of the muscarinic acetylcholine receptor m4
A C1-C4, C1-C4- technology, applied in the field of positive allosteric modulators of muscarinic acetylcholine receptor M4, can solve problems such as limiting clinical utility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0315] Example 1. General Amine Synthesis
[0316] The following are exemplary syntheses of two of the amines used to prepare the compounds disclosed herein.
[0317]
[0318] 4-(2-Hydroxypropan-2-yl)benzonitrile. To a solution of 4-iodobenzonitrile (10.0 g, 43.7 mmol, 1.0 equiv) in THF (218 mL) was added n-butyllithium (2.5 M in hexane, 22.7 mL) dropwise at -78 °C. 56.8 mmol, 1.3 eq) to keep the temperature below -70 °C. After 1 hour, acetone (32.0 mL, 436.6 mmol, 10.0 equiv) was added while maintaining the temperature below -70 °C. Remove the dry ice bath. After 16 h at room temperature, add saturated NH 4 Cl solution (100 mL), then EtOAc (250 mL) was added. The layers were separated. The aqueous layer was extracted with EtOAc (2 x 200 mL). The combined organic layers were washed with brine, dried (Na 2 SO 4 ), filtered and concentrated. The residue was purified by flash column chromatography on silica gel (0-60% EtOAc / Hexanes) to afford the title compound (4..8...
example 2
[0322] Example 2. N-[(3-fluoro-4-methoxy-phenyl)methyl]-2,4-dimethyl-quinoline-6-carboxamide (Compound 1)
[0323]
[0324] 6-Bromo-2,4-dimethylquinoline (A). A 40 mL reaction vial equipped with a Teflon septum and a magnetic stir bar was charged with 6-bromo-2-methylquinoline (555.2 mg, 2.5 mmol, 1.0 eq.), Ir catalyst (22.9 mg, 0.030 mmol), p-Toluenesulfonic acid monohydrate (951 mg, 5.0 mmol, 2.0 eq.), DMSO (10.0 mL, 0.25M), and methanol (20.0 mL, 0.50M). The mixture was degassed for 10 minutes by passing nitrogen with an exit needle. Ethyl-2-mercaptopropionate (16.3 uL, 0.130 mmol, 0.05 eq.) was added. The mixture was illuminated with a blue LED at room temperature under a micro fan. Complete conversion to the desired product was complete after 5 days where ethyl-2-mercaptopropionate (16.3uL, 0.130mmol, 0.05eq.) was added 3 more times. 1M NaOH solution (10.0 mL) and DCM (100.0 mL) were added. The organic layer was separated and washed with brine, washed with Na 2 S...
example 3
[0331] Example 3. N-[1-(5-chloro-2-methoxy-4-pyridyl)azetidin-3-yl]-2,4-dimethyl-quinoline-6-carboxamide (compound 2)
[0332]
[0333] N-(azetidin-3-yl)-2,4-dimethylquinoline-6-carboxamide (D). To a solution of 2,4-dimethylquinoline-6-carboxylic acid (C) (185.9 mg, 0.924 mmol, 1.0 eq.) in DMF (5.0 mL, 0.185 M) was added HATU (738 mg, 1.94 mmol, 2.1 eq.) and DIEA (0.804 mL, 4.62 mmol, 5.0 eq.). After stirring for 5 minutes, 1-Boc-3-(amino)azetidine (398 mg, 2.31 mmol, 2.5 eq.) was added. The reaction mixture was allowed to stir for 1 h. Using reverse phase HPLC (MeCN / H 2 O / 0.1% TFA) purified the crude material to provide the Boc-protected compound. Desired fractions were concentrated at 50°C using an air concentrator. Once the material was concentrated to dryness, the desired product with complete Boc deprotection was evident. Use a HF bond Elut-SCX column and use NH in MeOH 3 The crude material was purified by solution (7N) elution to afford the title compound (235...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com