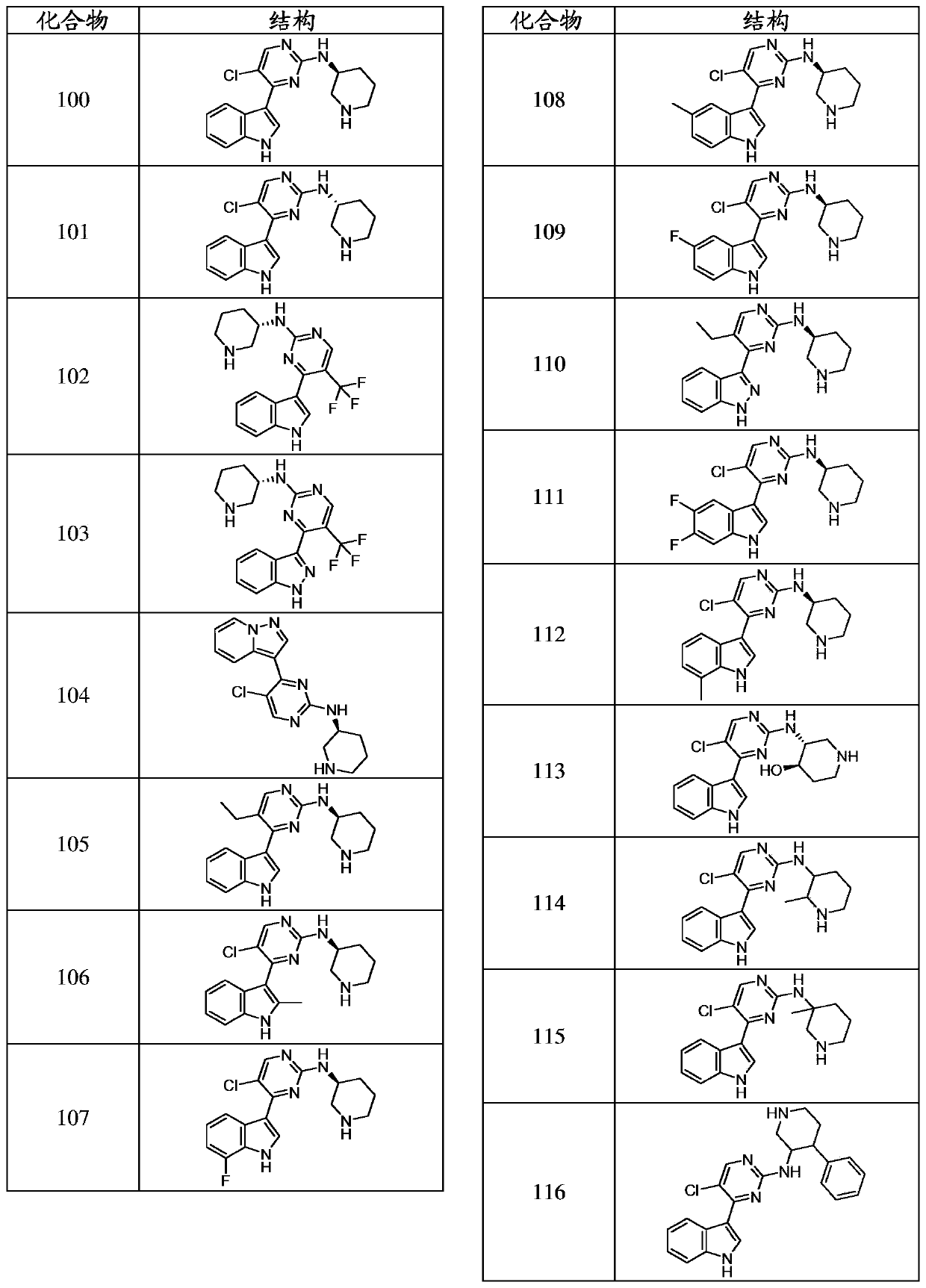
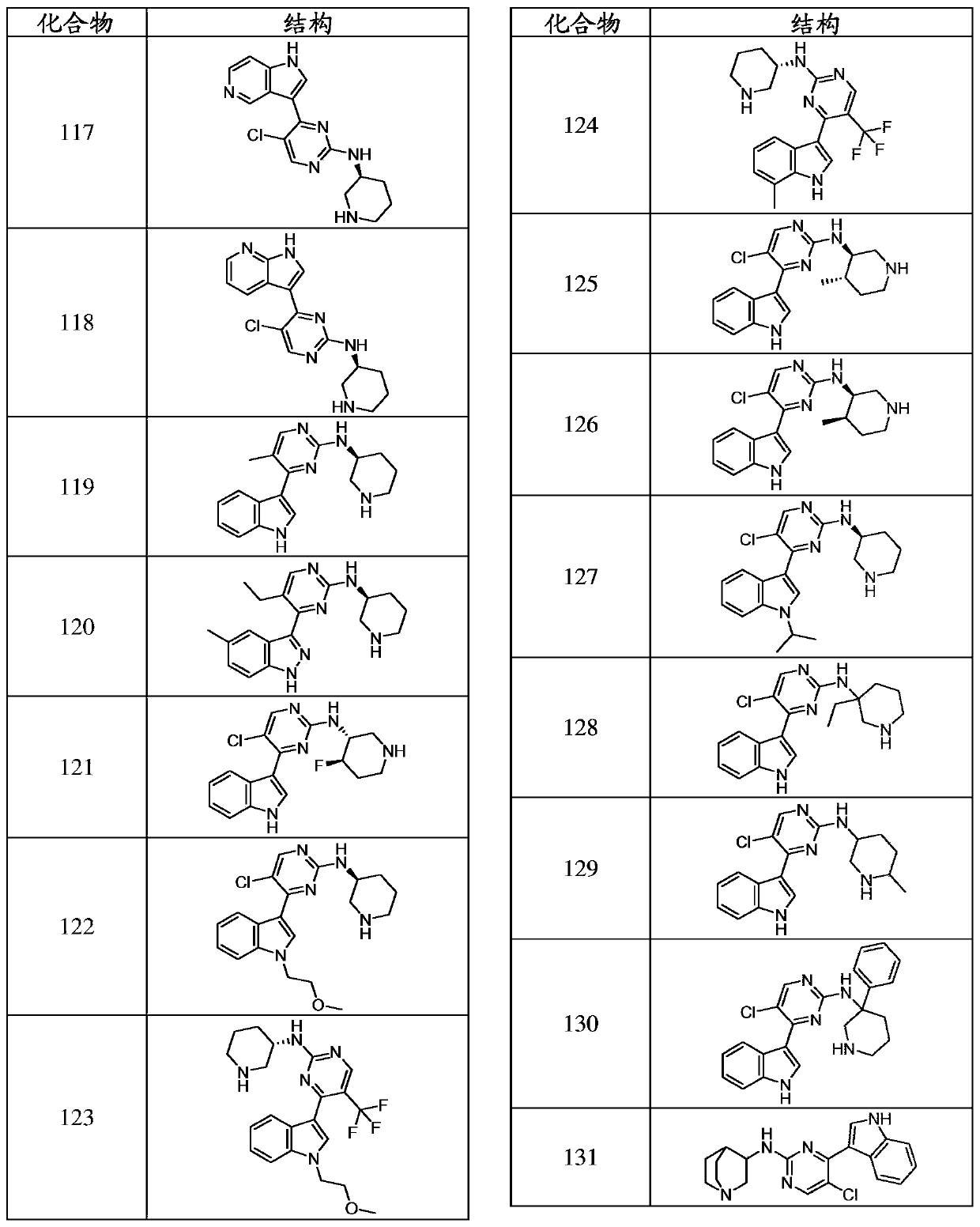
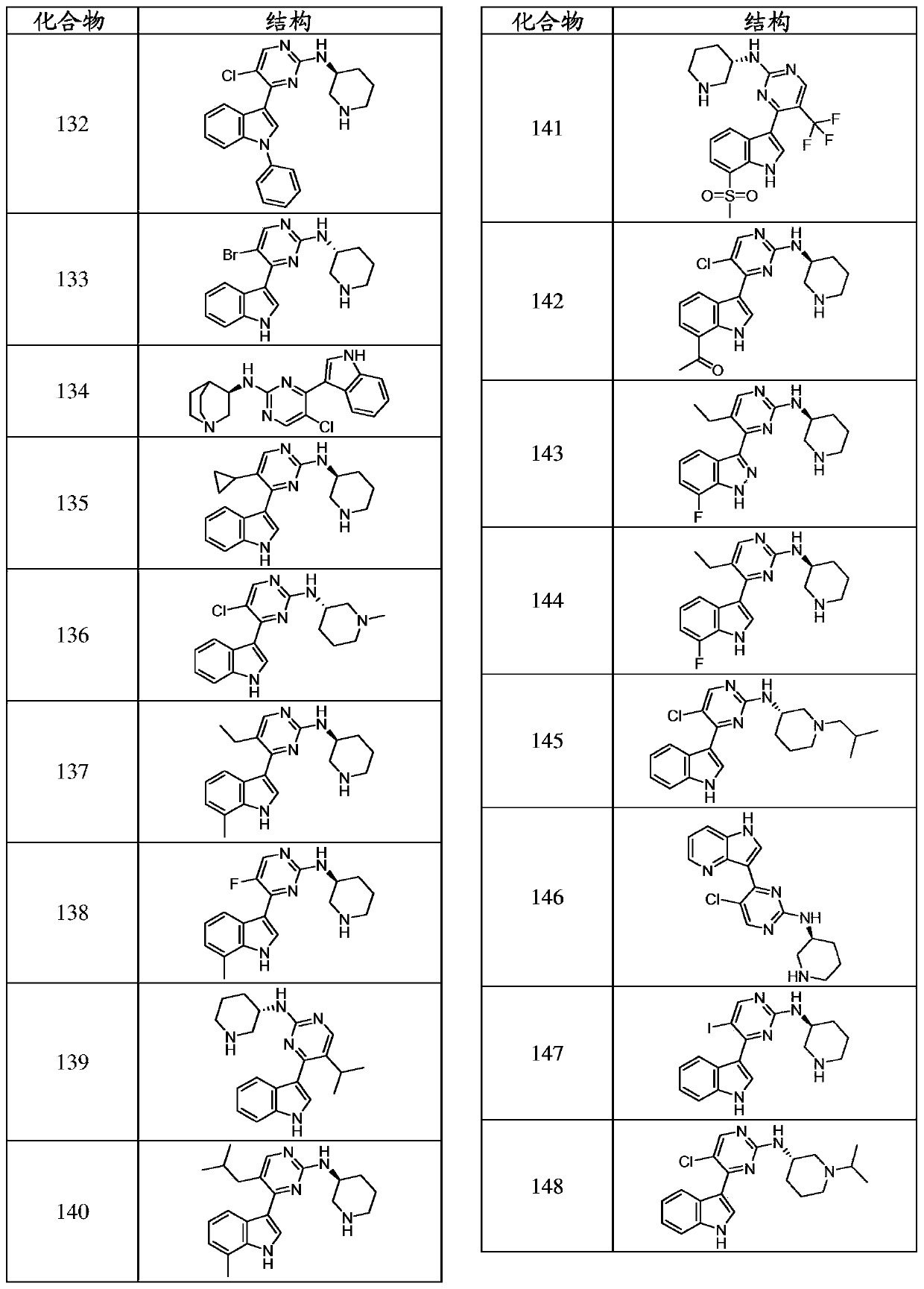
Inhibitors of cyclin dependnt kinase 7 (CDK7)
A C0-C6, C1-C6 technology, applied in the field of cyclin-dependent kinase 7 (CDK7) inhibitors, can solve problems that hinder the discovery of CDK7 selective inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0178] The pharmaceutical compositions described herein can be prepared by any method known in the art of pharmacology. Generally, such a preparation method comprises the following steps: combining a compound of formula (I) (ie, the "active ingredient") with a carrier and / or one or more other auxiliary agents, and then, if necessary and / or desired , forming and / or packaging the product into desired single- or multi-dose units.
[0179] The pharmaceutical compositions may be prepared, packaged and / or sold in bulk as a single unit dose and / or multiple single unit doses. As used herein, a "unit dose" is a discrete amount of a pharmaceutical composition containing a predetermined quantity of active ingredient. The amount of said active ingredient is usually equal to the dose of active ingredient to be administered to the subject and / or a simple fraction of that dose, for example, one-half or one-third of that dose.
[0180]The relative amounts of the active ingredients, pharmace...
Embodiment 1
[0223] Example 1 Synthesis of (S)-5-chloro-4-(5,6-difluoro-1H-indol-3-yl)-N-(piperidin-3-yl)pyrimidin-2-amine.
[0224] Step 1: 3-(2,5-Dichloropyrimidin-4-yl)-5,6-difluoro-1H-indole
[0225]
[0226] To a solution of 2,4,5-trichloropyrimidine (700 μL, 6.11 mmol) in dichloroethane (40 mL) was added aluminum chloride (940 mg, 7.05 mmol). The resulting suspension was stirred at 80°C for 30 minutes. The reaction mixture was then cooled to room temperature and 5,6-difluoroindole (1.00 g, 6.54 mmol) was added, and the resulting solution was stirred at 80 °C for 18 h. The reaction mixture was cooled to room temperature and crushed ice (15 mL) was added. The resulting slurry was stirred vigorously for 30 min, then further diluted with EtOAc (200 mL) and water (60 mL). The mixture was warmed to dissolve the suspended solids, the layers were separated and the aqueous layer was extracted with warm EtOAc (150 mL). The combined organic layers were washed with Na 2 SO 4 Dry, filter...
Embodiment 2
[0233] Example 2 Synthesis of (S)-N-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-yl)quinuclidin-3-amine (compound 131).
[0234]
[0235] (S)-N-(5-Chloro-4-(1-(phenylsulfonyl)-1H-indol-3-yl)pyrimidin-2-yl)quinuclidin-3-amine (50mg, 0.101 mmol) and NaOH 5M (1 mL, 5.00 mmol) in dioxane (1.0 mL) was heated at 70 °C for 6 h. The cooled mixture was then concentrated under reduced pressure. The crude residue was purified by reverse phase chromatography (C18, H 2 O / ACN+0.1%HCO 2 H, 0 to 100% gradient), then the title compound (24 mg, 0.067 mmol, 67% yield) was obtained as a white solid after lyophilization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com