High-molecular-weight furyl aromatic polyamide, preparation method and application thereof
A furan-based aromatic polyamide, high molecular weight technology, applied in the direction of one-component polyamide rayon, one-component synthetic polymer rayon, wet spinning, etc. The mechanical properties and mechanical properties of the amide product have no problems such as actually obtaining polymers, and achieve excellent thermodynamic properties and mechanical properties, simple process, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1: the high molecular weight furyl polyamide of preparation formula (III)
[0080] In an argon-protected glove box, 100 ml of N-methylpyrrolidone was added to a 250-ml three-necked round-bottomed flask equipped with a mechanical stirrer, an argon inlet, and an addition port, and stirred under mechanical stirring in an argon atmosphere. 20 g of 2,5-furandicarboxylic acid chloride were added. Then, the temperature was controlled by a water bath at a temperature of 20° C., and 20.8 g of 4,4-diaminodiphenyl ether was added under stirring, and 0.8 g of 2-(7-benzotriazole oxide)-N , N, N', N'-tetramethyluronium hexafluorophosphate, after continuing to stir for 5 hours, the reaction was terminated.
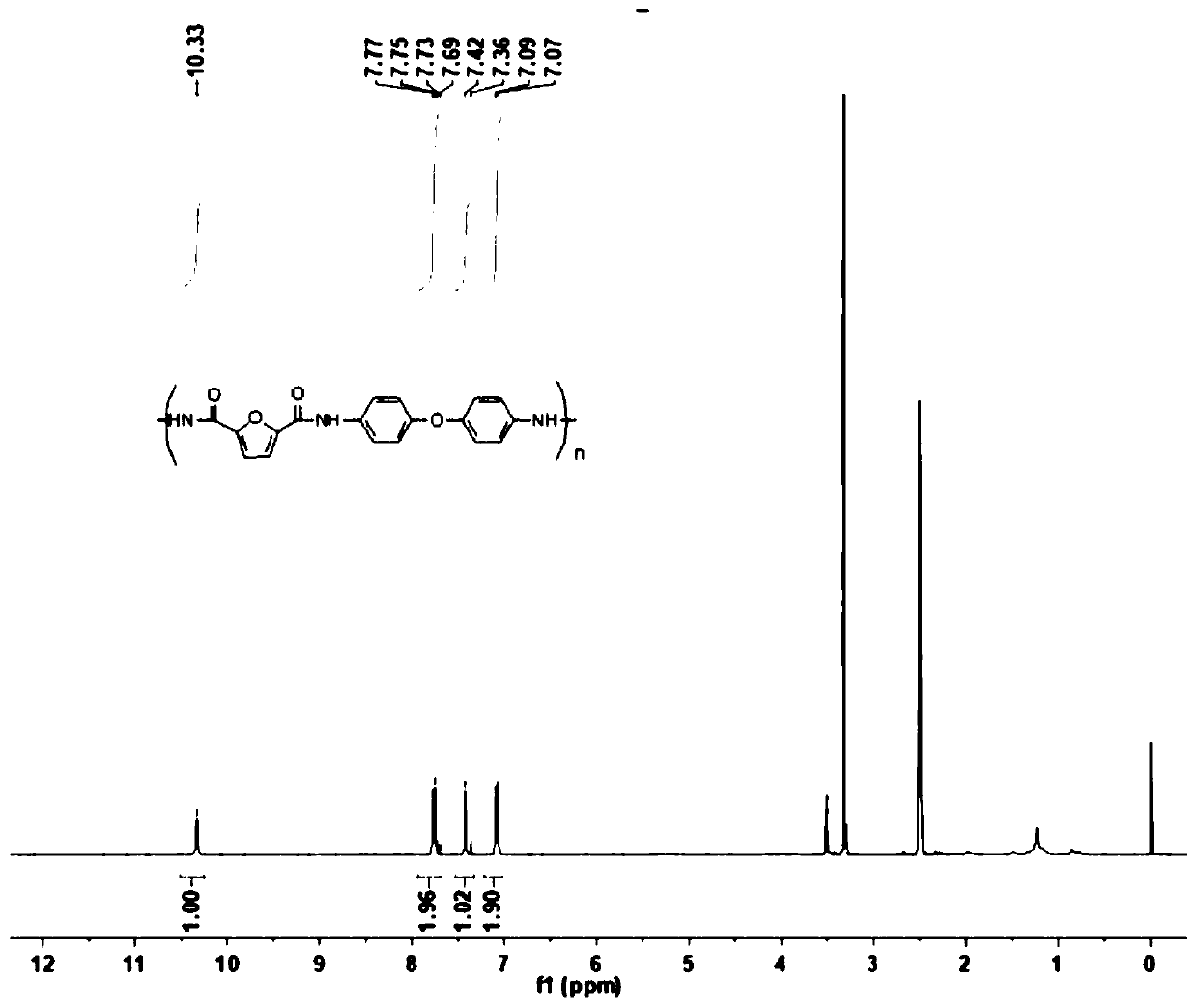
[0081] figure 1 The proton nuclear magnetic resonance spectrum of the furanyl aromatic polyamide polymer obtained according to the present embodiment 1 is shown, by figure 1 It was confirmed that the obtained polymer was a furyl aromatic polyamide polymer obtained by...
Embodiment 2
[0082] Embodiment 2: the high molecular weight furyl polyamide of preparation formula (IV)
[0083] In an argon-protected glove box, 100 ml of N-methylpyrrolidone was added to a 250-ml three-necked round-bottomed flask equipped with a mechanical stirrer, an argon inlet, and an addition port, and stirred under mechanical stirring in an argon atmosphere. 10 g of 2,5-furandicarboxylic acid chloride and 8.6 g of terephthalic acid were added. Then, the temperature was controlled by a water bath at a temperature of 20° C., and 10.4 g of 4,4-diaminodiphenyl ether and 11.25 g of p-phenylenediamine were added under stirring, while 0.8 g of 2-(7-phenylene oxide was added. and triazole)-N, N, N', N'-tetramethyluronium hexafluorophosphate, and continued stirring for 5 hours. After the reaction, sampling and analysis by gel permeation chromatography (GPC) instrument showed that the number average molecular weight of the obtained furyl polyamide was 321255, and the molecular weight distrib...
Embodiment 3
[0084] Embodiment 3: the high molecular weight furyl polyamide of preparation formula (V)
[0085] In an argon-protected glove box, 100 ml of N-methylpyrrolidone was added to a 250-ml three-necked round-bottomed flask equipped with a mechanical stirrer, an argon inlet, and an addition port, and stirred under mechanical stirring in an argon atmosphere. 20 g of 2,5-furandicarboxylic acid chloride were added. Then, the temperature was controlled by a water bath at a temperature of 20° C., and 10.4 g of 4,4-diaminodiphenyl ether and 11.25 g of p-phenylenediamine were added under stirring, and 0.8 g of 2-(7-benzoxyl oxide was added. Triazole)-N, N, N', N'-tetramethyluronium hexafluorophosphate, continued to stir and react for 5 hours. After the reaction was finished, the number average molecular weight of the obtained furanyl polyamide was 293768, and the molecular weight distribution coefficient was 1.50.
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com