Method for synthesis of tetrazolylacetic acid using hydrazine hydrate method
A technology of tetrazolium acetic acid and hydrazine hydrate, applied in the direction of organic chemistry and the like, can solve the problems of heavy environmental pollution, low safety factor and high production cost, and achieve the effects of high product purity, low production cost and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
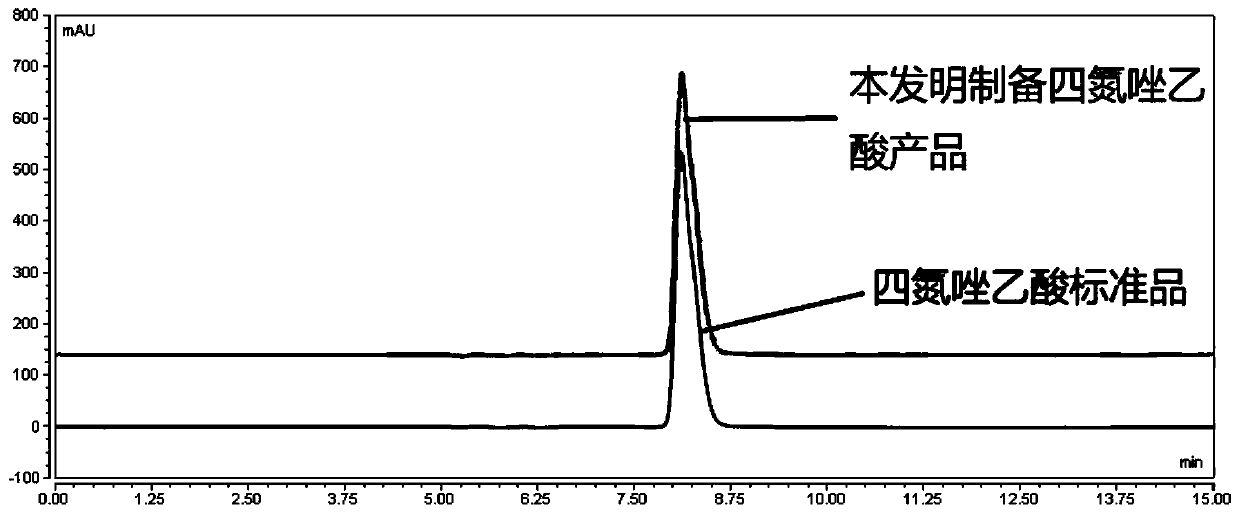
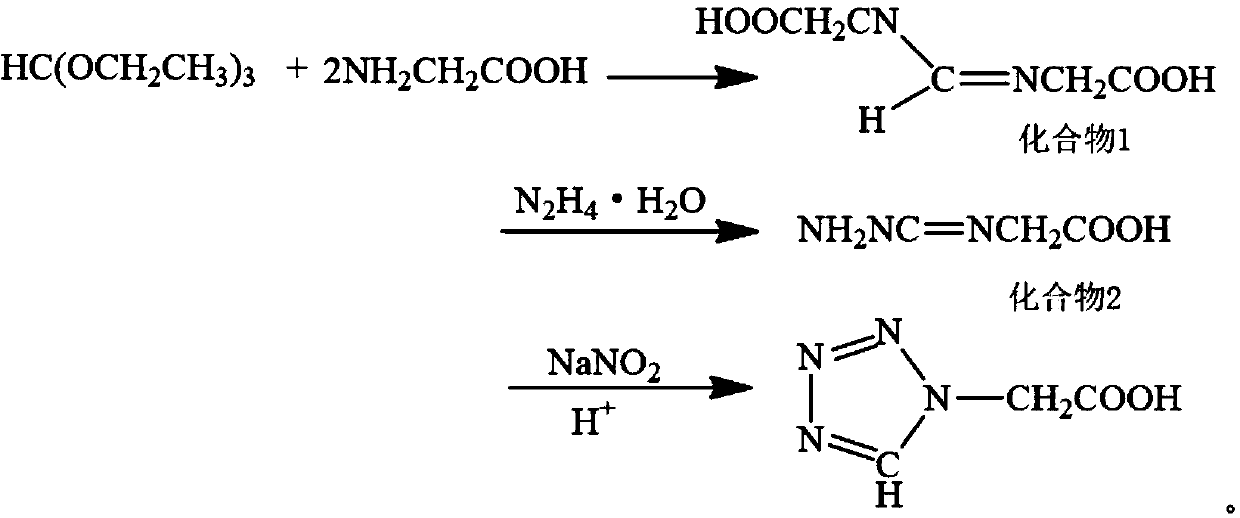
[0029] Add 44.46 g (0.3 mol) of triethyl orthoformate, 75.07 g (1 mol) of glycine and 300.25 g (5 mol) of glacial acetic acid into a 1000 mL reaction flask and react under reflux at 25° C. for 1 h. Add 29.5 g (0.5 mol) of 85% hydrazine hydrate to the above system, and heat under reflux at 55° C. for 3 h. Then add 41.40 g (0.6 mol) of sodium nitrite solid, and after reacting at 60 ° C for 2 hours, add 25 g (0.25 mol) of 98% concentrated sulfuric acid solution dropwise therein. , crystals were precipitated, and dried to obtain 30.2 g of tetrazoleacetic acid. Yield: 78.62%, content 99.6% (HPLC), melting point: 127-129°C.
Embodiment 2
[0031] 74.1 g (0.5 mol) of triethyl orthoformate, 75.07 g (1 mol) of glycine and 480.4 g (8 mol) of glacial acetic acid were added to a 1000 mL reaction flask and reacted under reflux at 45° C. for 3 h. Add 47.12 g (0.8 mol) of 85% hydrazine hydrate to the above system, and heat under reflux at 75° C. for 1 h. Then add 41.40 g (0.6 mol) of sodium nitrite solid, and after reacting at 60 ° C for 2 hours, add 25 g (0.25 mol) of 98% concentrated sulfuric acid solution dropwise therein. , crystals were precipitated, and dried to obtain 50.62 g of tetrazoleacetic acid. Yield: 79.07%, content 99.6% (HPLC), melting point: 128-129°C.
Embodiment 3
[0033] 74.1 g (0.5 mol) of triethyl orthoformate, 75.07 g (1 mol) of glycine and 480.4 g (8 mol) of glacial acetic acid were added to a 1000 mL reaction flask and reacted under reflux at 25° C. for 1 h. Add 47.12 g (0.8 mol) of 85% hydrazine hydrate to the above system, and heat under reflux at 75° C. for 3 h. Then add 55.20 g (0.8 mol) of sodium nitrite solid, and after reacting at 60 ° C for 2 hours, add 50 g (0.5 mol) of 98% concentrated sulfuric acid solution dropwise therein. , crystals were precipitated, and dried to obtain 50.9 g of tetrazoleacetic acid. Yield: 79.5%, content 99.6% (HPLC), melting point: 128-129°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com