Treatment of respiratory infection with a tlr2 agonist
An agonist, respiratory technology, applied in the field of prevention or treatment of respiratory diseases, can solve the problems of morbidity, mortality and high burden of health care costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
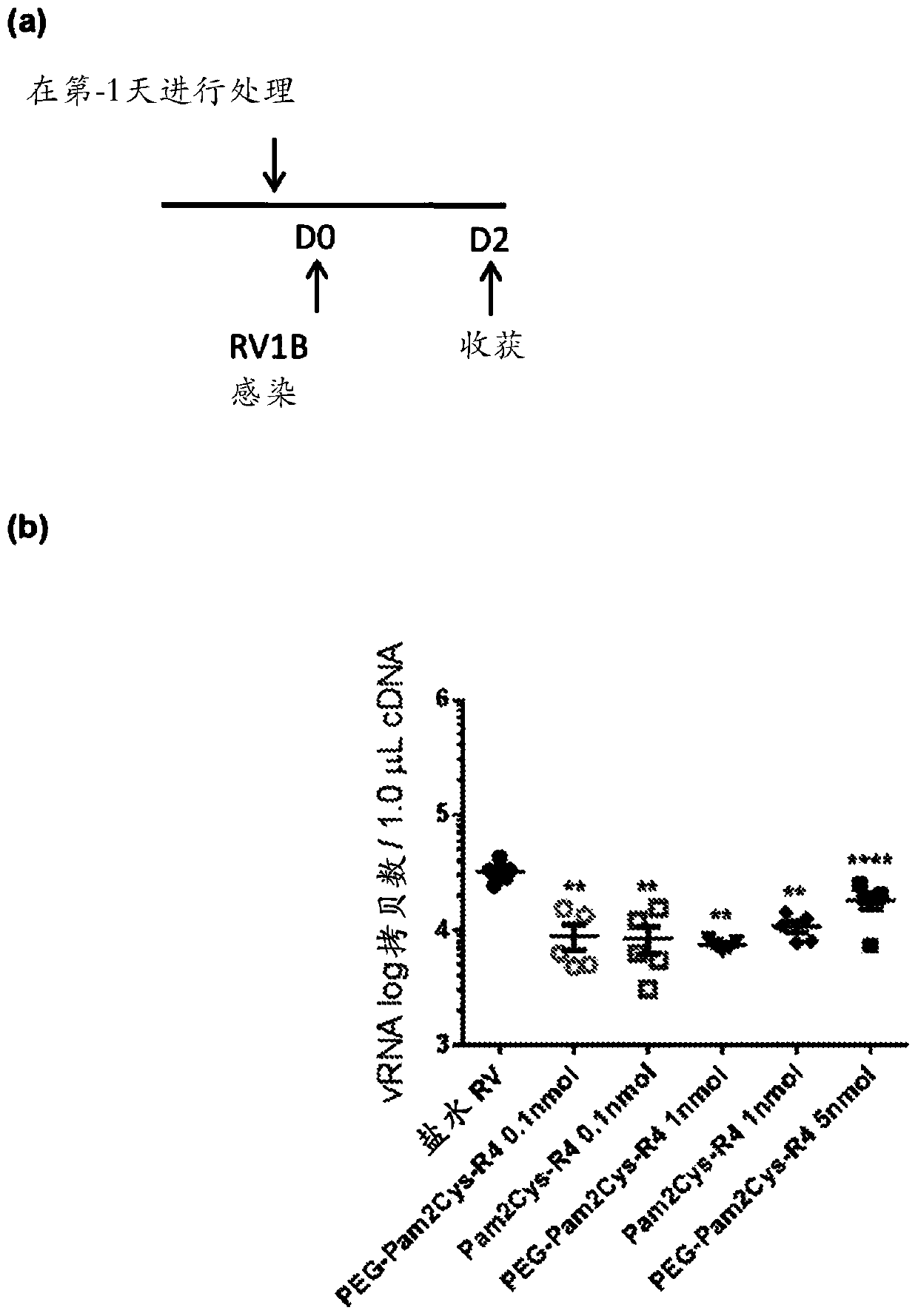
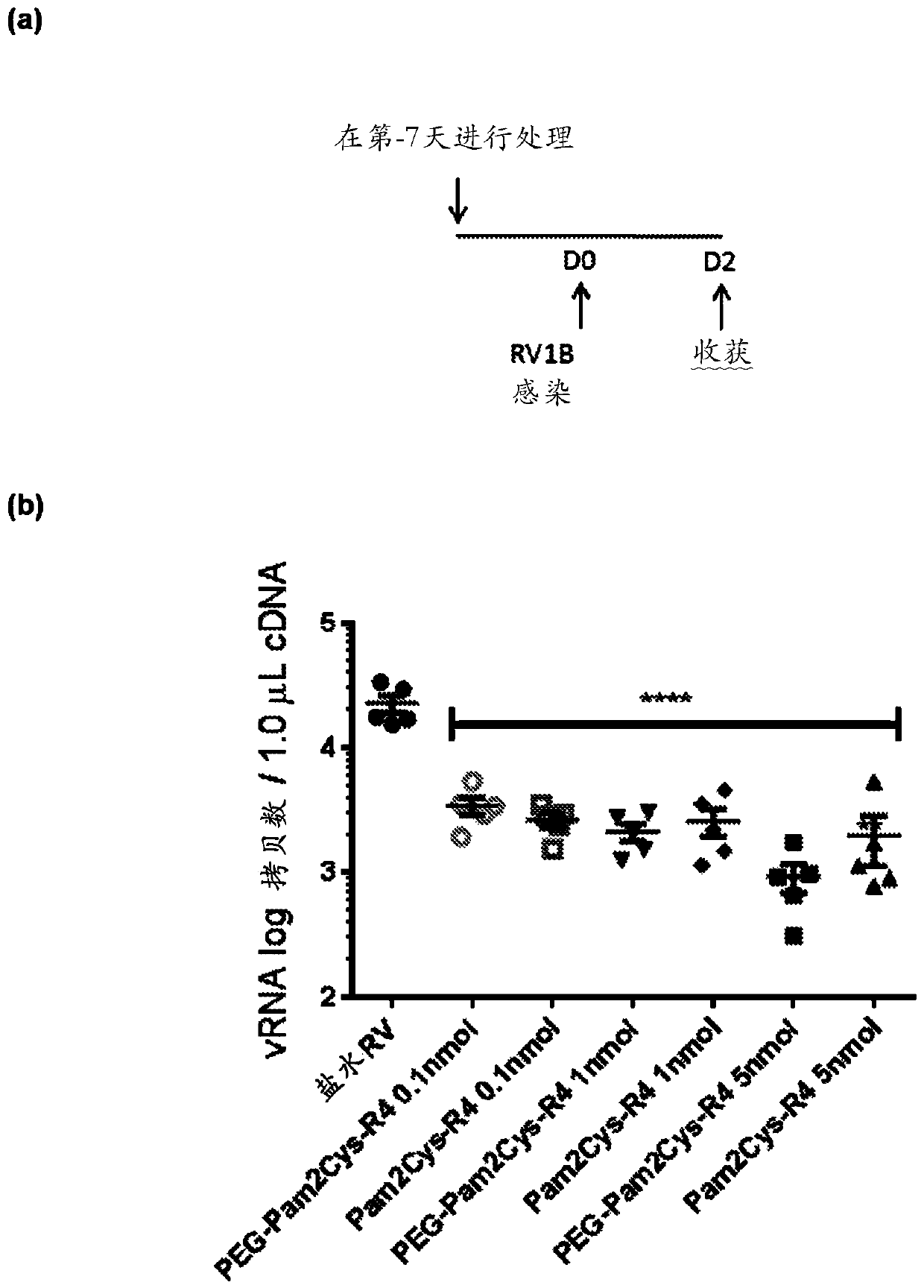
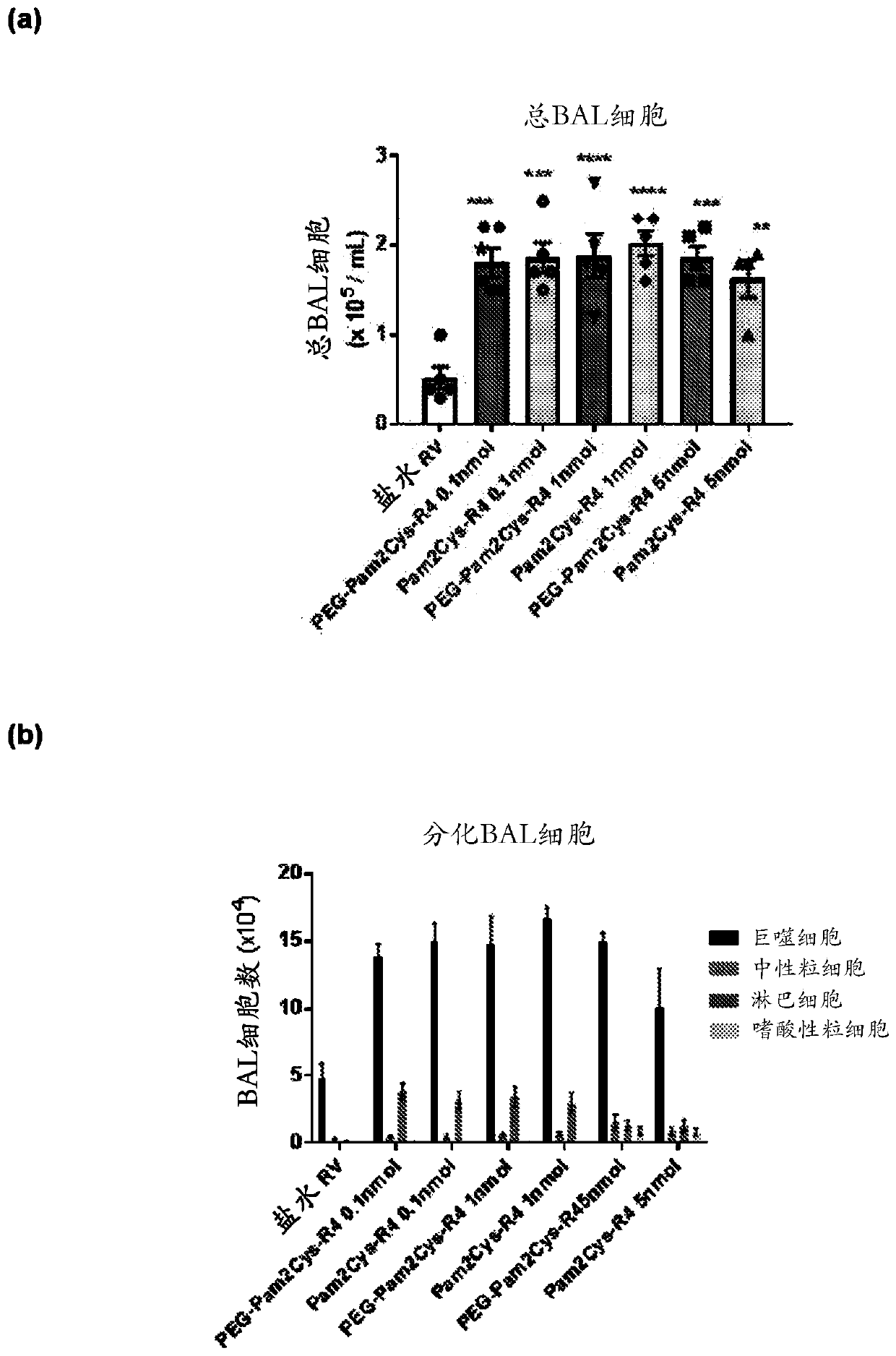
[0551] Inhibition of rhinovirus infection in a mouse model
[0552] This study was performed to determine whether activation of the innate immune system by TLR2 agonists reduces viral load and virus-induced inflammation during rhinovirus infection in mice.
[0553] animal
[0554] Female 6-8 week old BALB / c mice were used for all studies. Each group contained 5 mice. Following treatment or challenge procedures, mice were monitored daily for body weight changes and behavioral or physical changes, as specified in the Animal Ethics Approval for Project A-2016-605. At the time of sample collection, all mice were sacrificed by intraperitoneal administration of sodium pentobarbital. All mice were housed in individually ventilated cages at the HMRI Bioresources facility with no more than four mice per cage. Observe mice daily and maintain a health checklist from the beginning of each study.
[0555] Mouse Surgery and Treatment
[0556] Rhinovirus serotype 1B was initially purif...
example 2
[0613] Protective and therapeutic effects of TLR2 agonists against rhinovirus infection in primary asthmatic bronchial epithelial cells
[0614] This study was performed to determine whether TLR2 agonist treatment or prevention reduces viral load and virus-induced immune mediators in air-liquid interface (ALI)-differentiated human asthmatic bronchial epithelial cells during rhinovirus infection.
[0615] Air-liquid interface differentiation of primary bronchial epithelial cells from COPD patients
[0616] Primary bronchial epithelial cells obtained from 6 patients with mild to moderate persistent asthma ( Figure 12 a) Grown to confluence (passage 3) in T75 flasks and differentiated at the air-liquid interface (ALI). Briefly, primary cells were grown in submerged monolayer cultures in complete BEGM (Lonza) with growth factor supplements and then in 12-well plates with ALI-initial medium Seed until confluence (at least three days in both top and bottom compartments) with 2×10 ...
example 3
[0656] Synthesis of INNA-003 and INNA-006
[0657] Synthesis of INNA-003 and INNA-006
[0658] Reagents: Solid support: TentaGel S RAM resin (substitution factor 0.24 mmol / g; Rapp Polymere, Tübingen, Germany). Amino acid derivatives: Fmoc-Gly-OH, Fmoc-Ser(tBu)-OH, Fmoc-homo-Ser(tBu)-OH, Fmoc-homo-Ser(tBu)-OH from Merck, Darmstadt, Germany -Ser(PO(OBzl)OH)-OH, Fmoc-Thr(tBu)-OH, Fmoc-NH-(PEG) 3 -COOH, Fmoc-NH-(PEG) 5 -COOH, Fmoc-NH-(PEG) 11 -COOH, Fmoc-NH-(PEG) 27 -COOH.
[0659]
[0660] NOTE: Merck Cat. No. 851024 was used to generate the following as "INNA-003" (also referred to herein as Pam2Cys-SS-PEG) and "INNA-006" (also referred to herein as Pam2Cys-S-PEG) PEG) structure.
[0661] INNA-003:
[0662]
[0663] INNA-006 or Compound (1):
[0664]
[0665] Acylation: Use 4-fold molar excess of Fmoc amino acid, O-benzotriazole-N,N,N',N'-tetramethyl-uronium hexafluorophosphate (HBTU) and 6-fold molar excess in all acylation steps Molar excess of diisoprop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com