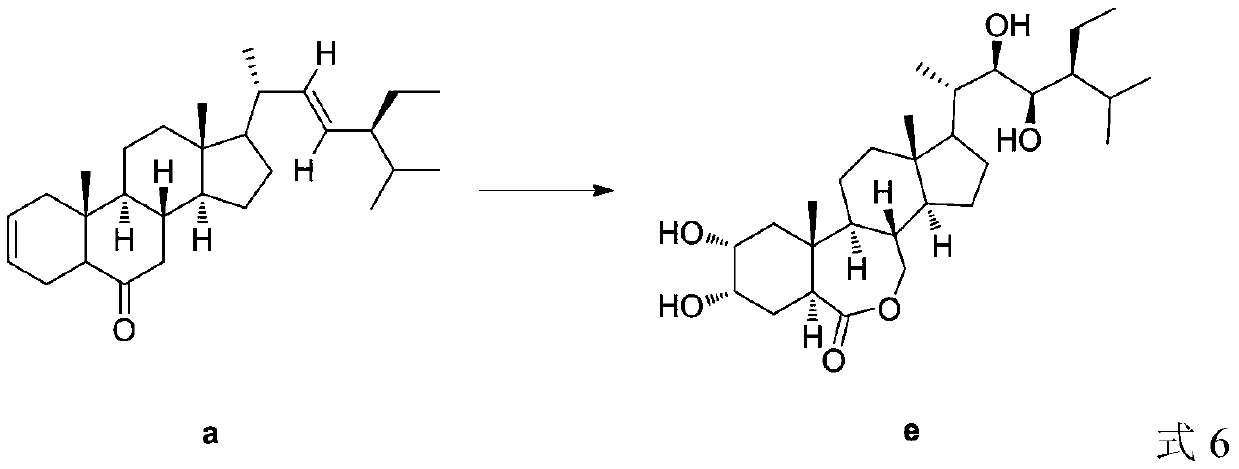
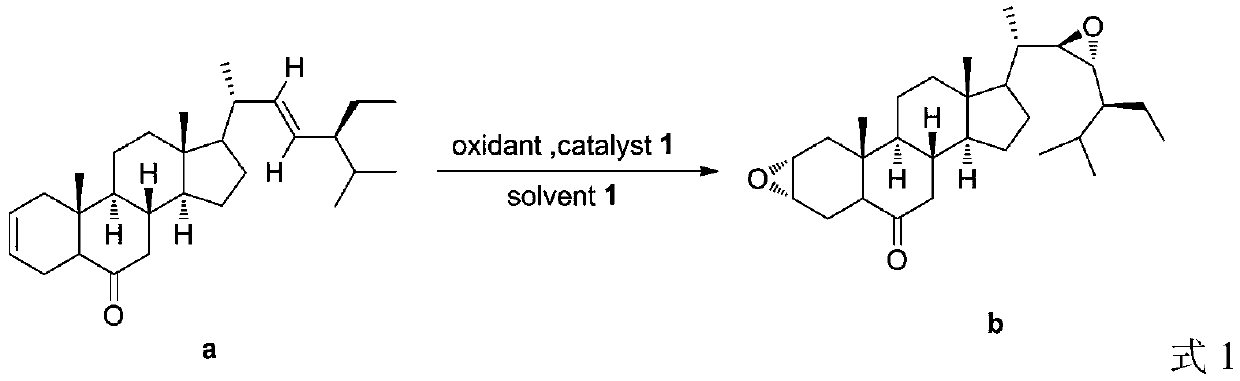
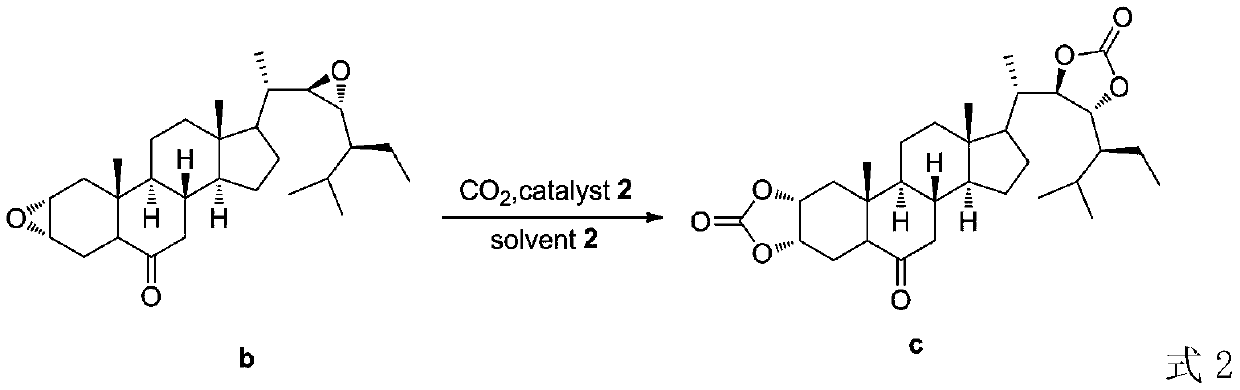
A kind of preparation method of 28-homobrasinolide
A technology of lactone and intermediate, which is applied in the field of preparation of 28-homobrassinolide, can solve the problems of complicated operation, difficult separation of products, pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add (2,22)-diene-24S-ethyl-5α-cholest-6-one (410.67g, 1mol), Cat 1 (0.5mol%), water in a reactor equipped with a thermometer and a stirrer (830ml), add hydrogen peroxide (37.41g) under stirring at 0°C, TLC detects that the reaction is complete, extract with dichloromethane, wash with saturated brine, recover dichloromethane to obtain 404.16g of intermediate b for use, with a yield of 91.3%. Intermediate b (404.16g), TBAB / GO (32.6g) and water (1210ml) were sequentially added to the reactor, carbon dioxide was introduced at room temperature until the reaction was complete, TBAB / GO was recovered by centrifugal separation, and washed with water to obtain intermediate c (410.75g) for use, yield 86.0%. Put the obtained intermediate c and water (1230ml) into the reactor, slowly add m-chloroperoxybenzoic acid (134.6g) at 10°C, stir at room temperature for 40min, wash with water, extract with dichloromethane, recover the dichloromethane to obtain the intermediate d is ready for ...
Embodiment 2
[0033] Add (2,22)-diene-24S-ethyl-5α-cholester-6-one (410.67g, 1mol), Cat 1 (0.1mol%), water in a reactor equipped with a thermometer and a stirrer (1640ml), hydrogen peroxide (41.1g) was added with stirring at 0°C, TLC detected until the reaction was complete, extracted with dichloromethane, washed with saturated brine, and recovered dichloromethane to obtain 395.75g of intermediate b for use with a yield of 89.4%. Intermediate b (395.75g), TBAB / GO (39.6g) and water (1980ml) were sequentially added to the reactor, carbon dioxide was introduced at room temperature until the reaction was complete, TBAB / GO was recovered by centrifugation, and washed with water to obtain intermediate c (401.7g) for use, yield 84.7%. Put the obtained intermediate c and water (1500ml) into the reactor, slowly add m-chloroperoxybenzoic acid (130.6g) at 10°C, stir at room temperature for 40min, wash with water, extract with dichloromethane, recover the dichloromethane to obtain the intermediate d is ...
Embodiment 3
[0035] Add (2,22)-diene-24S-ethyl-5α-cholest-6-one (410.67g, 1mol), Cat 2 (0.3mol%), water in a reactor equipped with a thermometer and a stirrer (1230ml), hydrogen peroxide (132.2g) was added under stirring at 30°C, TLC detected until the reaction was complete, extracted with dichloromethane, washed with saturated brine, recovered dichloromethane to obtain 370.7g of intermediate b for use, with a yield of 83.0%. Intermediate b (401.1g), TBAB / GO (80.2g) and water (4010ml) were sequentially added to the reactor, carbon dioxide was introduced at 50°C until the reaction was complete, TBAB / GO was recovered by centrifugal separation, and washed with water to obtain intermediate c (423.6g) for use, the yield was 88.1%. Put the obtained intermediate c and water (2000ml) into the reactor, slowly add m-chloroperoxybenzoic acid (137.7g) at 10°C, stir at room temperature for 40min, wash with water, extract with dichloromethane, recover the dichloromethane to obtain the intermediate d is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com