Composition for removing tumor immunosuppression and application thereof
A technology of immunosuppression and composition, applied in the fields of immunology and medicine, can solve the problems of unsatisfactory clinical effect, failure of DC vaccine to induce anti-tumor T cells, bankruptcy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0064] [Preparation method of engineered antigen-presenting cells]
[0065] The third aspect of the present invention provides a method for preparing engineered antigen-presenting cells, the engineered antigen-presenting cells are used to relieve tumor immunosuppression, which includes introducing the composition described in the first aspect into the antigen-presenting cells, Thereby engineering antigen-presenting cells.
[0066] In certain embodiments, the methods of the invention comprise the steps of:
[0067] (1) constructing a nucleic acid capable of producing components (a) to (e);
[0068] (2) isolate peripheral blood mononuclear cells from venous blood, and induce differentiation to obtain antigen-presenting cells; and
[0069] (3) introducing the nucleic acid of step (1) into the antigen-presenting cell of step (2), and culturing the antigen-presenting cell under conditions suitable for expression of the nucleic acid.
[0070] In a preferred embodiment, the method...
preparation example
[0074] This preparation example is for the preparation of DNA and mRNA encoding antigens and immune checkpoint inhibitors
[0075] 1. Preparation of DNA and mRNA Constructs
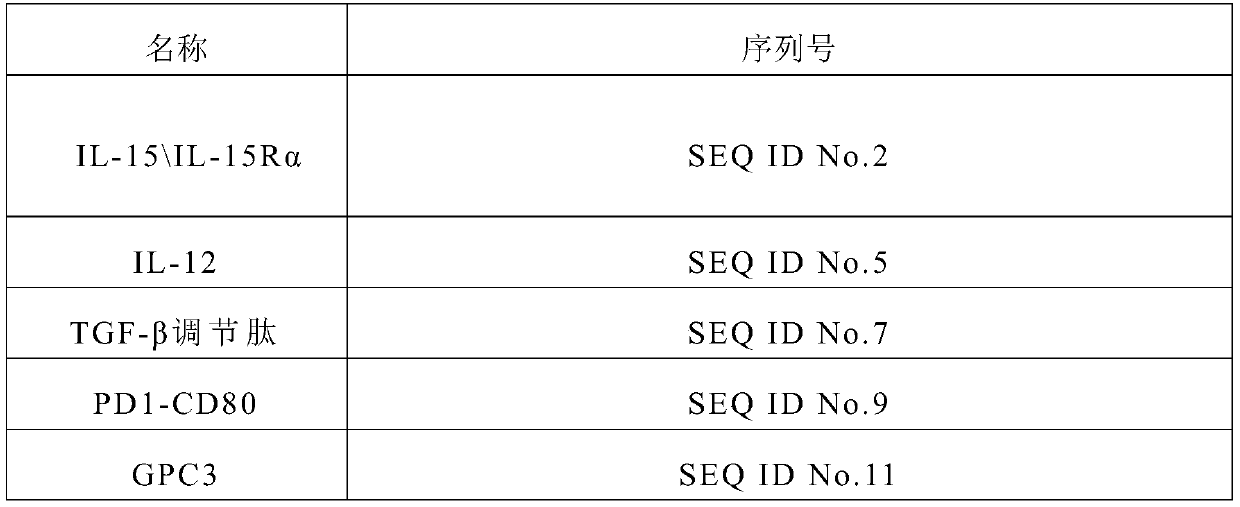
[0076] The DNA sequences used to produce the mRNAs encoding IL12, IL15, IL15Rα and TGF-β regulatory peptides and PD1-CD80 of the present invention were respectively constructed, and used for subsequent in vitro transcription reactions. Following the coding sequence is a polyadenosine segment. The DNA sequence information is shown in Table 1 below.
[0077] In addition, the coding sequence of human tumor antigen GPC3 for in vitro sensitization is constructed. The coding sequence of GPC3 in the present invention consists of the sequence shown in SEQ ID No.11, and the amino acid sequence consists of the sequence shown in SEQ ID No.12. The sequence of GPC3 is available through the Genebank database. In this example, the antigen disclosed in CN107583042A was used.
[0078] Table-1 DNA sequence list
[007...
Embodiment 1
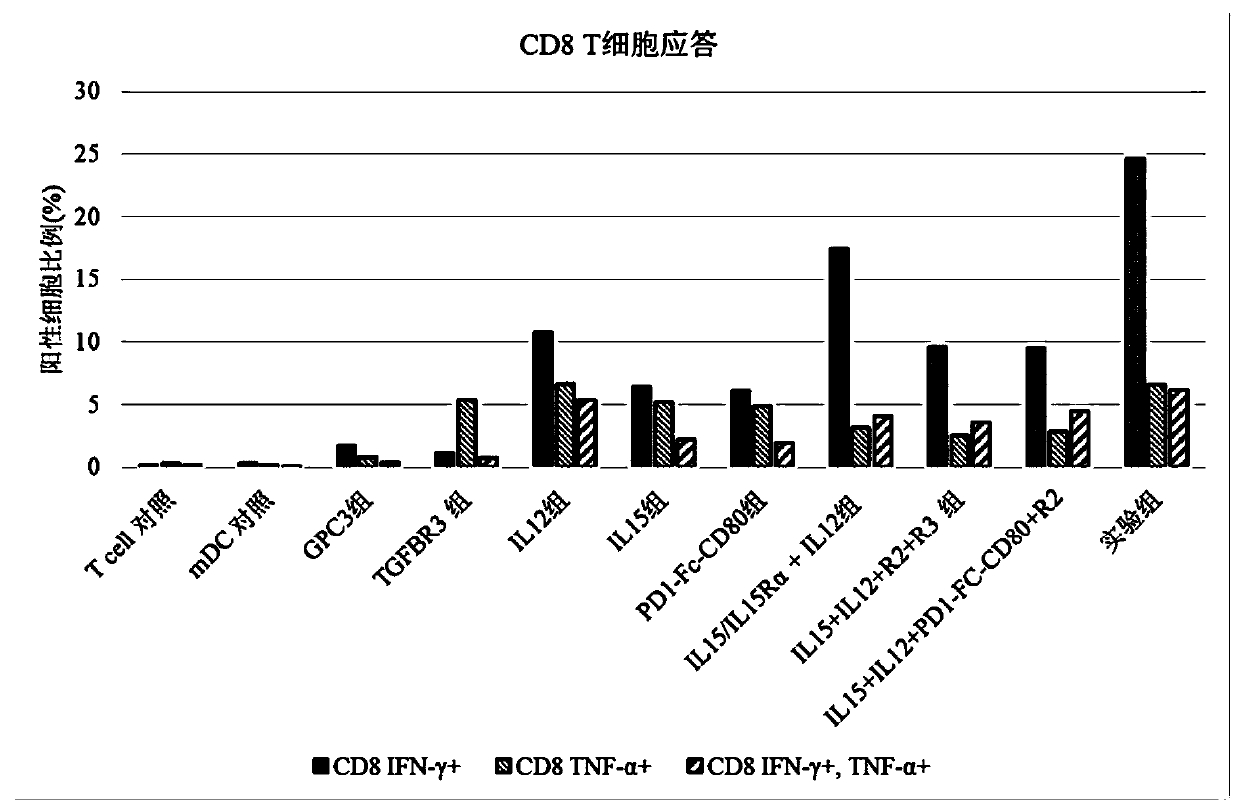
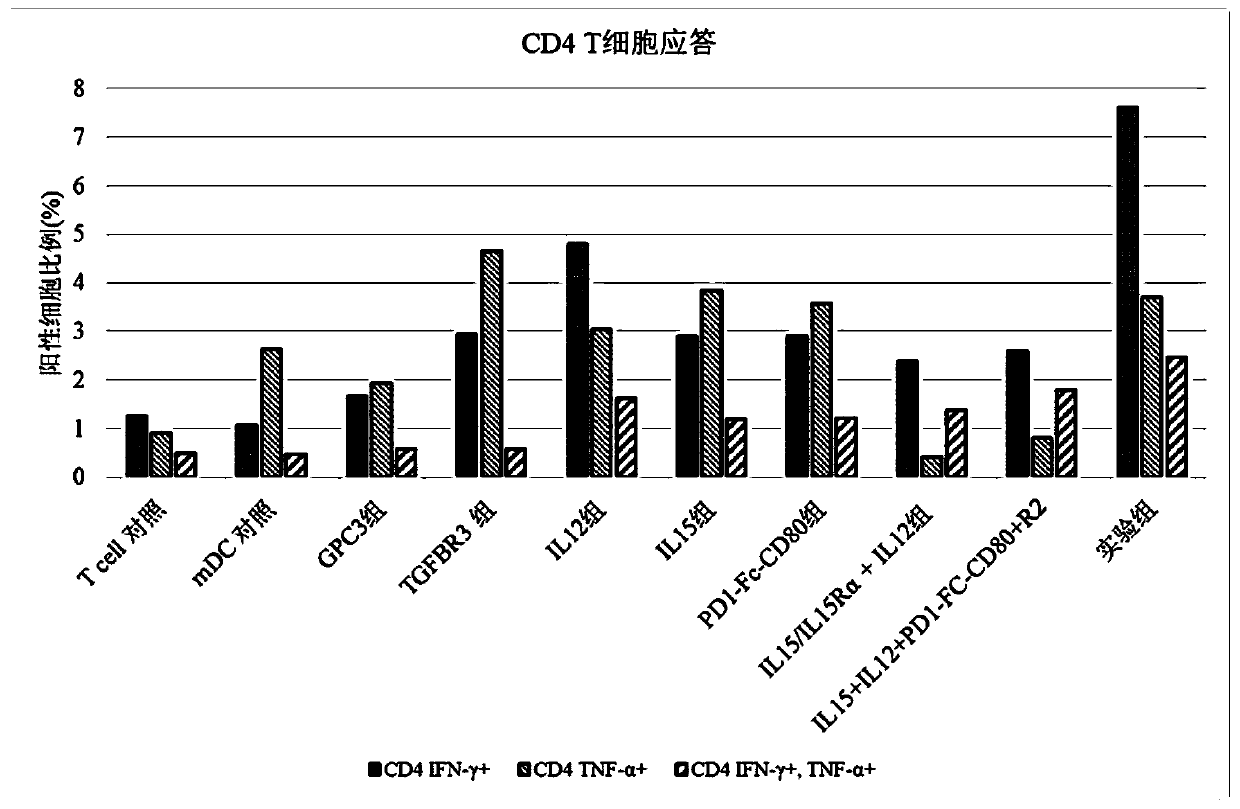
[0083] This example is used to study the effect of the composition of the present invention on T cell response.
[0084] 1. Induction culture of DC cells in vitro
[0085] Aseptically extract 50ml of venous blood from patients with hepatocellular carcinoma, separate peripheral blood mononuclear cells with lymphocyte separation medium in an ultra-clean workbench, add mononuclear cells to AIM-V medium, and place them in 37°C, 5% CO 2 Incubate in an incubator to allow monocytes to adhere to the wall. After 2h, the non-adherent cells were removed, and the adherent cells were added to iDC medium (GM-CSF with a final concentration of 800U / mL and IL-4 at 500U / mL were added to the AIM-V medium), and placed at 37°C for 5 %CO 2 Cultured in the incubator for 6 days. Transfer half of the cell culture medium to a centrifuge tube, collect the cells by centrifugation at 500g, remove the supernatant, and add an equal volume of fresh mDC medium (configuration of fresh medium for mDC: add AI...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com